Key Points
Mortality rates were higher with reduced-intensity regimens after haploidentical relative donor than with MUD transplantation.
Higher grade 3 and 4 acute GVHD occurred after haploidentical relative donor than with MUD transplantation.
Abstract
Posttransplant cyclophosphamide (PTCy) graft-versus-host disease (GVHD) prophylaxis has enabled haploidentical (Haplo) transplantation to be performed with results similar to those after matched unrelated donor (MUD) transplantation with traditional prophylaxis. The relative value of transplantation with MUD vs Haplo donors when both groups receive PTCy/calcineurin inhibitor/mycophenolate GVHD prophylaxis is not known. We compared outcomes after 2036 Haplo and 284 MUD transplantations with PTCy GVHD prophylaxis for acute leukemia or myelodysplastic syndrome in adults from 2011 through 2018. Cox regression models were built to compare outcomes between donor types. Recipients of myeloablative and reduced-intensity regimens were analyzed separately. Among recipients of reduced-intensity regimens, 2-year graft failure (3% vs 11%), acute grades 2 to 4 GVHD (hazards ratio [HR], 0.70; P = .022), acute grades 3 and 4 GVHD (HR, 0.41; P = .016), and nonrelapse mortality (HR, 0.43; P = .0008) were lower after MUD than with Haplo donor transplantation. Consequently, disease-free (HR, 0.74; P = .008; 55% vs 41%) and overall (HR, 0.65; P = .001; 67% vs 54%) survival were higher with MUD than with Haplo transplants. Among recipients of myeloablative regimens, day-100 platelet recovery (95% vs 88%) was higher and grades 3 and 4 acute (HR, 0.39; P = .07) and chronic GVHD (HR, 0.66; P = .05) were lower after MUD than with Haplo donor transplantation. There were no differences in graft failure, relapse, nonrelapse mortality, and disease-free and overall survival between donor types with myeloablative conditioning regimens. These data extend and confirm the importance of donor-recipient HLA matching for allogeneic transplantation. A MUD is the preferred donor, especially for transplantations with reduced-intensity conditioning regimens.
Introduction
Although allogeneic hematopoietic cell transplantation (HCT) remains the most important curative modality for hematologic malignancy, an HLA-matched sibling or unrelated donor (MUD) is not always readily available, particularly for ethnic minorities and multiethnic families.1 This problem has led to the expansion of the donor pool to include alternative donor sources such as HLA-haploidentical (Haplo) relatives, HLA-mismatched unrelated donors, and HLA-matched or mismatched cord blood.2 Posttransplantation cyclophosphamide (PTCy)-containing GVHD prophylaxis, pioneered at Johns Hopkins, has revolutionized Haplo HCT with acceptable rates of engraftment, graft-versus-host disease (GVHD), relapse, and survival.3,4 This effect was initially thought to be due to the tolerizing effect of PTCy on alloreactive donor T lymphocytes.5 Subsequent studies have also alluded to the preservation of regulatory T cells and the impaired functionality of effector T cells, which are critical to the pathophysiology of GVHD.6 The natural presumption that PTCy could lower GVHD after HLA-matched transplantation led to single-arm trials testing the hypothesis in the setting of HLA-matched sibling and MUD transplantation.7,8 A phase 2 trial (BMT CTN 1203) compared 3 novel GVHD prophylaxis regimens (PTCy/tacrolimus/mycophenolate mofetil, tacrolimus/methotrexate/bortezomib and tacrolimus/methotrexate/maraviroc) to the accepted standard tacrolimus/methotrexate prophylaxis for reduced-intensity–conditioning MUD HCT.9 In that trial, occurrences of grades 3 and 4 acute and chronic GVHD were lower and GVHD-free, relapse-free survival was higher in recipients of PTCy than with tacrolimus/methotrexate GVHD prophylaxis.9 An analysis from the Center for International Blood and Marrow Transplant Research (CIBMTR) that compared recipients of MUD HCT who received a calcineurin inhibitor (CNI) and methotrexate or mycophenolate GVHD prophylaxis to recipients of Haplo HCT with PTCy GVHD prophylaxis, showed higher rates of acute and chronic GVHD but comparable nonrelapse mortality, relapse, and survival after myeloablative and reduced-intensity conditioning regimens.10 The interpretation of the results of this study was limited, in that the observed differences in acute and chronic GVHD risks could have been explained by graft type, as peripheral blood was the predominant graft for MUD HCT, and bone marrow was the predominant graft for Haplo HCT.10
Clinical practice has changed in recent years with increasing use of PTCy GVHD prophylaxis for MUD HCT and an increasing number of peripheral blood stem cell grafts for Haplo HCT. Therefore, the objective of the current study was to compare outcomes after MUD to Haplo HCT with PTCy as the backbone of GVHD prophylaxis in patients with acute myeloid leukemia (AML), acute lymphoblastic leukemia, and myelodysplastic syndrome (MDS). The fundamental question as to whether a MUD is preferred to a Haplo donor when both donors are readily available is unknown and would be best addressed in a randomized trial. Such a trial is challenging, as any potential trial subject must have both donors to be eligible for randomization, which would lengthen the duration of the trial. In the absence of a trial that randomizes eligible subjects to a MUD or Haplo donor, we undertook a controlled observational study to address this question by reviewing data reported to the CIBMTR.
Methods
Patients
Data were obtained from the CIBMTR, a working group of transplant centers that submit data on standardized reporting forms, with patients being observed longitudinally. Patients underwent transplantation in the United States at 111 centers from 2011 through 2018. Fifty-three centers performed transplants with both donor types (MUD and Haplo), 56 centers used only Haplo donors, and 2 centers used only MUDs. Included were patients aged ≥18 years with AML, acute lymphoblastic leukemia in first or second complete remission, or MDS. Haplo donors were mismatched at ≥2 HLA loci, and MUDs were matched at the allele level at HLA-A, -B, -C and -DRB1. Patients underwent myeloablative or reduced-intensity conditioning regimens, as previously defined,11 and received T-cell replete grafts. All patients had received PTCy and CNI/mycophenolate mofetil for GVHD prophylaxis. Seventy-nine patients who had received PTCy/CNI without mycophenolate mofetil were excluded. Other exclusions included patients who underwent transplantation in the third complete remission or in relapse, those with MDS that transformed to AML, and those who underwent in vivo T-cell depletion (n = 39) and received CD34+-selected peripheral blood grafts. Patients provided written informed consent, and the Institutional Review Board of the National Marrow Donor Program approved the study.
Outcomes
Overall survival was the primary end point. Other end points included hematopoietic recovery, acute and chronic GVHD, relapse, nonrelapse mortality, and disease-free survival. Neutrophil recovery was defined as achieving an absolute neutrophil count (ANC) ≥0.5 × 109/L for 3 consecutive days. Graft failure was defined as failure to achieve ANC ≥0.5 × 109/L or a decline in ANC to <0.5 × 109/L without recovery, after having achieved ANC ≥0.5 × 109/L; or myeloid donor chimerism (<5%); or having a second transplant.12 Disease relapse, progression, and death were treated as events. Nonrelapse mortality was defined as time to death without relapse or progression. Relapse was defined as molecular, cytogenetic, or morphologic recurrence of hematologic malignancy. Disease-free survival was defined as being alive without relapse. Grades 2 to 4 acute and chronic GVHD were assigned according to previously described criteria.13,14
Statistical analysis
A separate analysis was performed for those who underwent myeloablative or reduced-intensity conditioning regimens. The incidence of neutrophil recovery and graft failure were calculated by using the cumulative incidence estimator.15 Multivariate analyses were performed with Cox proportional hazards models for acute and chronic GVHD, relapse, nonrelapse mortality, and disease-free and overall survival, to examine the effect of donor type with adjustment for age, sex, race, performance score, HCT comorbidity score, cytomegalovirus (CMV) serostatus, disease risk index, transplant conditioning regimen (TBI- vs non-TBI regimens), graft type, and transplant period (Tables 1 and 2).16 Age was treated as a binary variable (≤55 vs >55 years). The age cutoff at 55 years was determined statistically by the minimum P-value approach. To determine the optimal age cutoff, we used a series of 2-sample tests for multiple possible candidate dichotomizations of age. For each candidate cutoff, an appropriate Cox model with a binary covariate for age was constructed, and the P value for the Wald test was obtained. The optimal age cutoff was defined as the candidate cutoff with the lowest P value. A stepwise model building approach was adopted, and variables that attained a P ≤ .05 were retained in the final model with the exception of the variable for donor type, which was held in the final model regardless of its level of significance. The incidence of acute and chronic GVHD and the probabilities of relapse and nonrelapse mortality and disease-free and overall survival were calculated from the final Cox model.17,18 Transplant center effect on survival was tested using the frailty model.19 All P values were 2 sided, and analyses were performed in SAS, version 9.4 (Cary, NC).
Characteristics of patients who received reduced-intensity regimens
| Variable . | Donor type . | P . | |
|---|---|---|---|
| Haplo PTCy/CNI/MMF . | MUD PTCy/CNI/MMF . | ||
| No. of patients | 1211 | 187 | |
| Median age (range), y | 62 (18-81) | 65 (20-80) | .01 |
| Age group | |||
| ≤55 | 371 (31) | 24 (13) | |
| >55 | 840 (69) | 163 (87) | |
| Race | <.0001 | ||
| White | 874 (72) | 177 (95) | |
| Other | 337 (28) | 10 (5) | |
| Sex | .42 | ||
| Male | 718 (56) | 105 (56) | |
| Female | 493 (41) | 82 (44) | |
| Performance score | .21 | ||
| 90-100 | 636 (53) | 111 (59) | |
| ≤80 | 549 (45) | 73 (39) | |
| Not reported | 26 (2) | 3 (2) | |
| Comorbidity score | .27 | ||
| ≤2 | 603 (50) | 85 (46) | |
| ≥3 | 608 (50) | 102 (54) | |
| Recipient CMV serostatus | .16 | ||
| Negative | 372 (31) | 70 (37) | |
| Positive | 837 (69) | 117 (63) | |
| Not reported | 2 (< 1) | — | |
| Disease | .77 | ||
| Acute myeloid leukemia | 724 (60) | 113 (60) | |
| Acute lymphoblastic leukemia | 212 (18) | 29 (16) | |
| Myelodysplastic syndrome | 275 (23) | 45 (24) | |
| Disease risk index | .07 | ||
| Low/intermediate risk | 954 (79) | 149 (80) | |
| High/very high risk | 224 (18) | 36 (19) | |
| Not reported | 33 (3) | 2 (1) | |
| Interval from diagnosis to HCT, median (IQR), mo | |||
| Low/intermediate risk | 6 (4-10) | 6 (4-9) | .87 |
| High/very high risk | 9 (5-19) | 8 (5-11) | .08 |
| Conditioning regimen | <.0001 | ||
| TBI | 1140 (94) | 88 (47) | |
| TBI/fludarabine/cyclophosphamide | 1040 | 70 | |
| TBI/fludarabine | 29 | 6 | |
| TBI/melphalan | 71 | 12 | |
| Non-TBI | 71 (6) | 99 (53) | |
| Fludarabine/busulfan | 12 | 38 | |
| Fludarabine/melphalan | 59 | 61 | |
| Graft type | <.0001 | ||
| Bone marrow | 535 (44) | 30 (16) | |
| Peripheral blood | 676 (56) | 157 (84) | |
| Transplant period | .01 | ||
| 2011-2014 | 256 (21) | 25 (13) | |
| 2015-2018 | 955 (79) | 162 (87) | |
| Variable . | Donor type . | P . | |
|---|---|---|---|
| Haplo PTCy/CNI/MMF . | MUD PTCy/CNI/MMF . | ||
| No. of patients | 1211 | 187 | |
| Median age (range), y | 62 (18-81) | 65 (20-80) | .01 |
| Age group | |||
| ≤55 | 371 (31) | 24 (13) | |
| >55 | 840 (69) | 163 (87) | |
| Race | <.0001 | ||
| White | 874 (72) | 177 (95) | |
| Other | 337 (28) | 10 (5) | |
| Sex | .42 | ||
| Male | 718 (56) | 105 (56) | |
| Female | 493 (41) | 82 (44) | |
| Performance score | .21 | ||
| 90-100 | 636 (53) | 111 (59) | |
| ≤80 | 549 (45) | 73 (39) | |
| Not reported | 26 (2) | 3 (2) | |
| Comorbidity score | .27 | ||
| ≤2 | 603 (50) | 85 (46) | |
| ≥3 | 608 (50) | 102 (54) | |
| Recipient CMV serostatus | .16 | ||
| Negative | 372 (31) | 70 (37) | |
| Positive | 837 (69) | 117 (63) | |
| Not reported | 2 (< 1) | — | |
| Disease | .77 | ||
| Acute myeloid leukemia | 724 (60) | 113 (60) | |
| Acute lymphoblastic leukemia | 212 (18) | 29 (16) | |
| Myelodysplastic syndrome | 275 (23) | 45 (24) | |
| Disease risk index | .07 | ||
| Low/intermediate risk | 954 (79) | 149 (80) | |
| High/very high risk | 224 (18) | 36 (19) | |
| Not reported | 33 (3) | 2 (1) | |
| Interval from diagnosis to HCT, median (IQR), mo | |||
| Low/intermediate risk | 6 (4-10) | 6 (4-9) | .87 |
| High/very high risk | 9 (5-19) | 8 (5-11) | .08 |
| Conditioning regimen | <.0001 | ||
| TBI | 1140 (94) | 88 (47) | |
| TBI/fludarabine/cyclophosphamide | 1040 | 70 | |
| TBI/fludarabine | 29 | 6 | |
| TBI/melphalan | 71 | 12 | |
| Non-TBI | 71 (6) | 99 (53) | |
| Fludarabine/busulfan | 12 | 38 | |
| Fludarabine/melphalan | 59 | 61 | |
| Graft type | <.0001 | ||
| Bone marrow | 535 (44) | 30 (16) | |
| Peripheral blood | 676 (56) | 157 (84) | |
| Transplant period | .01 | ||
| 2011-2014 | 256 (21) | 25 (13) | |
| 2015-2018 | 955 (79) | 162 (87) | |
Data are no. of patients (percentage of study group), unless otherwise stated.
IQR, interquartile range.
Characteristics of patients who received myeloablative conditioning regimens
| Variable . | Donor type . | P . | |
|---|---|---|---|
| Haplo PTCy/CNI/MMF . | MUD PTCy/CNI/MMF . | ||
| No. of patients | 825 | 97 | |
| Age, median (range), y | 45 (18-75) | 50 (18-71) | .002 |
| Age group | |||
| ≤55 y | 591 (72) | 55 (57) | |
| >55 y | 234 (28) | 42 (43) | |
| Race | .001 | ||
| White | 567 (69) | 85 (88) | |
| Other | 258 (31) | 12 (12) | |
| Sex | .52 | ||
| Male | 462 (56) | 51 (53) | |
| Female | 363 (44) | 46 (47) | |
| Performance score | .60 | ||
| 90-100 | 461 (56) | 57 (59) | |
| ≤80 | 348 (42) | 37 (38) | |
| Not reported | 16 (2) | 3 (3) | |
| Comorbidity score | .56 | ||
| ≤2 | 451 (55) | 50 (52) | |
| ≥3 | 374 (45) | 47 (48) | |
| Recipient CMV serostatus | .04 | ||
| Negative | 245 (30) | 41 (42) | |
| Positive | 577 (70) | 56 (58) | |
| Not reported | 3 (< 1) | __ | |
| Disease | .01 | ||
| Acute myeloid leukemia | 453 (55) | 47 (48) | |
| Acute lymphoblastic leukemia | 268 (32) | 27 (28) | |
| Myelodysplastic syndrome | 104 (13) | 23 (24) | |
| Disease risk index | .39 | ||
| Low/intermediate risk | 627 (76) | 77 (79) | |
| High/very high risk | 168 (20) | 19 (20) | |
| Not reported | 30 (4) | 1 (1) | |
| Interval from diagnosis to HCT, median (IQR), mo | |||
| Low/intermediate risk | 6 (4-9) | 6 (5-10) | .75 |
| High/very high risk | 12 (5-29) | 8 (5-14) | .08 |
| Conditioning regimen | .003 | ||
| TBI | 414 (50) | 33 (34) | |
| TBI/fludarabine | 360 | 15 | |
| TBI/cyclophosphamide | 39 | 4 | |
| TBI/other agents | 15 | 14 | |
| Non-TBI | 411 (50) | 64 (66) | |
| Busulfan/cyclophosphamide | 213 | 20 | |
| Fludarabine/busulfan | 153 | 43 | |
| Fludarabine/melphalan/thiotepa | 45 | 1 | |
| Graft type | .003 | ||
| Bone marrow | 216 (26) | 12 (12) | |
| Peripheral blood | 609 (74) | 85 (88) | |
| Transplant period | .006 | ||
| 2011-2014 | 151 (19) | 7 (7) | |
| 2015-2018 | 674 (81) | 90 (93) | |
| Variable . | Donor type . | P . | |
|---|---|---|---|
| Haplo PTCy/CNI/MMF . | MUD PTCy/CNI/MMF . | ||
| No. of patients | 825 | 97 | |
| Age, median (range), y | 45 (18-75) | 50 (18-71) | .002 |
| Age group | |||
| ≤55 y | 591 (72) | 55 (57) | |
| >55 y | 234 (28) | 42 (43) | |
| Race | .001 | ||
| White | 567 (69) | 85 (88) | |
| Other | 258 (31) | 12 (12) | |
| Sex | .52 | ||
| Male | 462 (56) | 51 (53) | |
| Female | 363 (44) | 46 (47) | |
| Performance score | .60 | ||
| 90-100 | 461 (56) | 57 (59) | |
| ≤80 | 348 (42) | 37 (38) | |
| Not reported | 16 (2) | 3 (3) | |
| Comorbidity score | .56 | ||
| ≤2 | 451 (55) | 50 (52) | |
| ≥3 | 374 (45) | 47 (48) | |
| Recipient CMV serostatus | .04 | ||
| Negative | 245 (30) | 41 (42) | |
| Positive | 577 (70) | 56 (58) | |
| Not reported | 3 (< 1) | __ | |
| Disease | .01 | ||
| Acute myeloid leukemia | 453 (55) | 47 (48) | |
| Acute lymphoblastic leukemia | 268 (32) | 27 (28) | |
| Myelodysplastic syndrome | 104 (13) | 23 (24) | |
| Disease risk index | .39 | ||
| Low/intermediate risk | 627 (76) | 77 (79) | |
| High/very high risk | 168 (20) | 19 (20) | |
| Not reported | 30 (4) | 1 (1) | |
| Interval from diagnosis to HCT, median (IQR), mo | |||
| Low/intermediate risk | 6 (4-9) | 6 (5-10) | .75 |
| High/very high risk | 12 (5-29) | 8 (5-14) | .08 |
| Conditioning regimen | .003 | ||
| TBI | 414 (50) | 33 (34) | |
| TBI/fludarabine | 360 | 15 | |
| TBI/cyclophosphamide | 39 | 4 | |
| TBI/other agents | 15 | 14 | |
| Non-TBI | 411 (50) | 64 (66) | |
| Busulfan/cyclophosphamide | 213 | 20 | |
| Fludarabine/busulfan | 153 | 43 | |
| Fludarabine/melphalan/thiotepa | 45 | 1 | |
| Graft type | .003 | ||
| Bone marrow | 216 (26) | 12 (12) | |
| Peripheral blood | 609 (74) | 85 (88) | |
| Transplant period | .006 | ||
| 2011-2014 | 151 (19) | 7 (7) | |
| 2015-2018 | 674 (81) | 90 (93) | |
Data are no, of patients (percentage of study group), unless otherwise stated.
IQR, interquartile range.
Results
Patient, disease, and transplant characteristics
Table 1 shows the characteristics of patients who underwent reduced-intensity conditioning regimens by donor type. All recipients of MUD and Haplo HCT received PTCy/CNI/mycophenolate for GVHD prophylaxis. Patient characteristics (sex, performance score, comorbidity, and CMV serostatus) did not differ between treatment groups, but recipients of Haplo HCT were younger (median age at transplantation was 62 years compared with 65 years for MUD recipients) and less likely to be White. AML was the predominant disease in both treatment groups. Most patients in both treatment groups had low/intermediate disease risk index. Bone marrow was more common for Haplo HCT than it was for MUD HCT, although peripheral blood was the predominant graft for both donor types. The predominant regimen for Haplo HCTs included low-dose TBI with fludarabine and cyclophosphamide. On the other hand, MUD HCTs were just as likely to use low-dose TBI with fludarabine and cyclophosphamide or an alkylating agent and fludarabine. The interval between diagnosis and transplantation did not differ by donor type. MUD HCTs were more common from 2015 through 2018 and accounted for 87% of such transplantations, compared with 79% of Haplo HCTs. The median follow-ups of recipients of Haplo and MUD HCTs were 29 (range, 3-101) and 25 (range, 5-94) months, respectively. Therefore, all outcomes were censored at 24 months.
Table 2 shows the characteristics of patients who underwent myeloablative conditioning regimens by donor type. All recipients of Haplo and MUD HCT received PTCy/CNI/mycophenolate for GVHD prophylaxis. Patient characteristics (sex, performance score, and comorbidity score) did not differ between treatment groups, but recipients of Haplo donor HCT were younger, more likely to be CMV seropositive, and less likely to be White. AML was the predominant disease in both treatment groups, but fewer patients with MDS received Haplo HCT. Most patients in both treatment groups had a low-to-intermediate disease risk index, and peripheral blood was the predominant graft. Recipients of Haplo HCT grafts were equally likely to undergo the TBI+fludarabine or alkylator+fludarabine regimen. Approximately two-thirds of recipients of MUD HCT had the alkylator+fludarabine regimen. MUD HCTs were more common from 2015 through 2018 and accounted for 90% of such transplantations. The median follow-ups of recipients of Haplo donor and MUD HCT were 25 (range, 3-96) and 12 months (range, 6-87) months, respectively. Therefore, outcomes were censored at 12 months.
Reduced-intensity conditioning regimen HCT
Hematopoietic recovery
Neutrophil and platelet recovery rates were lower after Haplo HCT (Table 3). The 2-year incidence of graft failure was higher after Haplo than with MUD HCT (Table 3).
Transplant outcomes by donor type
| Outcome . | Donor type . | P . | |
|---|---|---|---|
| Haplo, % (95% CI) . | MUD, % (95% CI) . | ||
| Reduced-intensity regimen | |||
| Day-28 neutrophil recovery | 90 (88-92) | 96 (93-98) | <.001 |
| Day-100 platelet recovery | 88 (86-90) | 95 (92-98) | <.001 |
| 2-y graft failure | 11 (8-13) | 3 (1-7) | <.001 |
| Day-100 grades 2 to 4 acute GVHD | 29 (27-32) | 29 (22-36) | .17 |
| Day-100 grades 3 and 4 acute GVHD | 9 (7-10) | 4 (2-8) | .02 |
| 2-y chronic GVHD | 27 (25-30) | 29 (22-36) | .70 |
| 2-y relapse | 42 (39-45) | 37 (30-45) | .22 |
| 2-y nonrelapse mortality | 16 (14-19) | 8 (5-13) | <.001 |
| 2-y disease-free survival | 41 (38-45) | 55 (47-62) | .002 |
| 2-y overall survival | 54 (51-57) | 67 (60-74) | .001 |
| Myeloablative regimen | |||
| Day-28 neutrophil recovery | 94 (92-95) | 96 (91-99) | .31 |
| Day-100 platelet recovery | 87 (85-89) | 93 (87-97) | <.0001 |
| 1-y graft failure | 4 (3-6) | 3 (1-8) | .60 |
| Day-100 grades 2 to 4 acute GVHD | 33 (30-37) | 32 (23-41) | .73 |
| Day-100 grades 3 and 4 acute GVHD | 10 (8-12) | 4 (1-9) | .02 |
| 1-y chronic GVHD | 33 (30-36) | 25 (17-34) | .09 |
| 1-y relapse | 19 (17-22) | 21 (23-41) | .74 |
| 1-y nonrelapse mortality | 15 (13-18) | 15 (8-23) | .97 |
| 1-y disease-free survival | 66 (62-69) | 65 (54-74) | .81 |
| 1-y overall survival | 75 (72-78) | 77 (68-85) | .59 |
| Outcome . | Donor type . | P . | |
|---|---|---|---|
| Haplo, % (95% CI) . | MUD, % (95% CI) . | ||
| Reduced-intensity regimen | |||
| Day-28 neutrophil recovery | 90 (88-92) | 96 (93-98) | <.001 |
| Day-100 platelet recovery | 88 (86-90) | 95 (92-98) | <.001 |
| 2-y graft failure | 11 (8-13) | 3 (1-7) | <.001 |
| Day-100 grades 2 to 4 acute GVHD | 29 (27-32) | 29 (22-36) | .17 |
| Day-100 grades 3 and 4 acute GVHD | 9 (7-10) | 4 (2-8) | .02 |
| 2-y chronic GVHD | 27 (25-30) | 29 (22-36) | .70 |
| 2-y relapse | 42 (39-45) | 37 (30-45) | .22 |
| 2-y nonrelapse mortality | 16 (14-19) | 8 (5-13) | <.001 |
| 2-y disease-free survival | 41 (38-45) | 55 (47-62) | .002 |
| 2-y overall survival | 54 (51-57) | 67 (60-74) | .001 |
| Myeloablative regimen | |||
| Day-28 neutrophil recovery | 94 (92-95) | 96 (91-99) | .31 |
| Day-100 platelet recovery | 87 (85-89) | 93 (87-97) | <.0001 |
| 1-y graft failure | 4 (3-6) | 3 (1-8) | .60 |
| Day-100 grades 2 to 4 acute GVHD | 33 (30-37) | 32 (23-41) | .73 |
| Day-100 grades 3 and 4 acute GVHD | 10 (8-12) | 4 (1-9) | .02 |
| 1-y chronic GVHD | 33 (30-36) | 25 (17-34) | .09 |
| 1-y relapse | 19 (17-22) | 21 (23-41) | .74 |
| 1-y nonrelapse mortality | 15 (13-18) | 15 (8-23) | .97 |
| 1-y disease-free survival | 66 (62-69) | 65 (54-74) | .81 |
| 1-y overall survival | 75 (72-78) | 77 (68-85) | .59 |
GVHD, relapse, NRM, and survival
Table 4 shows the risk of acute and chronic GVHD, nonrelapse mortality, relapse, and disease-free and overall survival by donor type. Compared with Haplo HCT, the risk of grades 2 to 4 and 3 and 4 acute GVHD was lower with MUD HCT. Chronic GVHD risk did not differ by donor type. The incidence of acute and chronic GVHD are shown in Table 3. Nonrelapse mortality risks were lower after MUD HCT, resulting in higher disease-free and overall survival than with Haplo HCT (Tables 3 and 4; Figure 1). Relapse risks did not differ by donor type. Both bone marrow and peripheral blood grafts were used, and peripheral blood use was substantially higher in MUD HCTs. Peripheral blood was associated with higher grade 2 and 4 acute (P = .98) and chronic GVHD (P = .07) and nonrelapse mortality (P = .24), but its effect was independent of donor type. We evaluated for effect of transplant center by using the frailty model and found no effect. We also performed a sensitivity analysis limited to centers that performed both Haplo and MUD HCTs. Consistent with the main analysis, compared with Haplo HCT, survival was higher after MUD HCT (hazards ratio [HR], 0.75; 95% confidence interval [CI], 0.57-0.98; P = .03). Data on infections were available for a subset of patients (∼25% of the study population). The 6-month incidence of fungal infection was higher after Haplo (10%; 95% CI, 8-13) than with MUD (1%; 95% CI, 0% to 5%; P < .001) transplantation. There were no differences in the incidence of viral and bacterial infections (data not shown). One hundred fifty-nine of 1211 (13%) recipients of Haplo HCT received donor leukocyte infusion or underwent a second HCT, compared with 16 of 187 (9%) recipients of MUD HCT (P = .08).
Reduced-intensity regimens: multivariate analysis of outcomes
| Outcome . | No. of events/evaluable . | HR (95% CI) . | P . |
|---|---|---|---|
| Grades 2 to 4 acute GVHD* | |||
| Haplo | 389/1171 | 1.00 | |
| MUD | 49/180 | 0.70 (0.52-0.95) | .022 |
| Grades 3 and 4 acute GVHD† | |||
| Haplo | 118/1167 | 1.00 | |
| MUD | 8/179 | 0.41 (0.20-0.85) | .016 |
| Chronic GVHD‡ | |||
| Haplo | 309/1195 | 1.00 | |
| MUD | 51/185 | 0.80 (0.60-1.09) | .15 |
| Relapse§ | |||
| Haplo | 512/1205 | 1.00 | |
| MUD | 72/187 | 0.99 (0.75-1.31) | .94 |
| Nonrelapse mortality║ | |||
| Haplo | 205/1205 | 1.00 | |
| MUD | 18/187 | 0.33 (0.19-0.57) | <.0001 |
| Disease-free survival¶ | |||
| Haplo | 717/1205 | 1.00 | |
| MUD | 90/187 | 0.74 (0.60-0.93) | .008 |
| Overall survival# | |||
| Haplo | 568/1211 | 1.00 | |
| MUD | 65/187 | 0.65 (0.50-0.84) | .001 |
| Outcome . | No. of events/evaluable . | HR (95% CI) . | P . |
|---|---|---|---|
| Grades 2 to 4 acute GVHD* | |||
| Haplo | 389/1171 | 1.00 | |
| MUD | 49/180 | 0.70 (0.52-0.95) | .022 |
| Grades 3 and 4 acute GVHD† | |||
| Haplo | 118/1167 | 1.00 | |
| MUD | 8/179 | 0.41 (0.20-0.85) | .016 |
| Chronic GVHD‡ | |||
| Haplo | 309/1195 | 1.00 | |
| MUD | 51/185 | 0.80 (0.60-1.09) | .15 |
| Relapse§ | |||
| Haplo | 512/1205 | 1.00 | |
| MUD | 72/187 | 0.99 (0.75-1.31) | .94 |
| Nonrelapse mortality║ | |||
| Haplo | 205/1205 | 1.00 | |
| MUD | 18/187 | 0.33 (0.19-0.57) | <.0001 |
| Disease-free survival¶ | |||
| Haplo | 717/1205 | 1.00 | |
| MUD | 90/187 | 0.74 (0.60-0.93) | .008 |
| Overall survival# | |||
| Haplo | 568/1211 | 1.00 | |
| MUD | 65/187 | 0.65 (0.50-0.84) | .001 |
Model adjusted for graft type (peripheral blood: HR, 1.34; 95% CI, 1.10-1.64; P = .004).
Model not adjusted; no significant factors.
Model adjusted for graft type (peripheral blood: HR, 2.14; 95% CI, 1.76-2.59; P < .0001) and conditioning regimen (non-TBI: HR, 0.67; 95% CI, 0.49-0.92; P = .015).
Model adjusted for disease risk index (high-risk: HR, 2.11; 95% CI, 1.74-2.56; P < .0001).
Model adjusted for age (>55 y: HR, 1.62; 95% CI, 1.17-2.24; P = .004), hematopoietic comorbidity index (≥3: HR, 1.70; 95% CI, 1.29-2.23; P = .0002), graft type (peripheral blood: HR, 1.39; 95% CI, 1.05-1.85; P = .02), and conditioning regimen (non-TBI: HR, 1.68; 95% CI, 1.11-2.55; P = .014).
Model adjusted for hematopoietic comorbidity index (≥3: HR, 1.26; 95% CI, 1.09-1.45; P = .001) and disease risk index (high-risk: HR, 2.16; 95% CI, 1.83-2.54; P < .0001).
Model adjusted for age (>55 y: HR, 1.43; 95% CI, 1.19-1.72; P = .0001), hematopoietic comorbidity index (≥ 3: HR, 1.32; 95% CI, 1.12-1.54; P = .0007), and disease risk index (high-risk: HR, 2.16; 95% CI, 1.81-2.59; P < .0001).
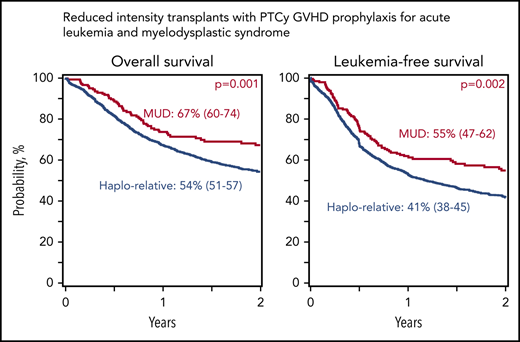
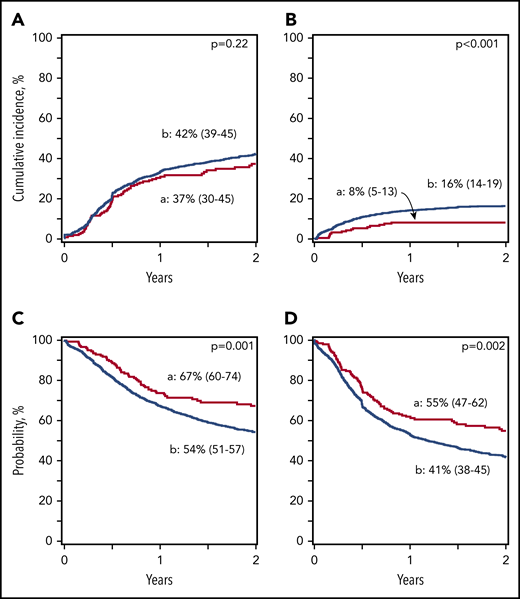
Reduced-intensity regimens: incidence of nonrelapse mortality, relapse, and disease-free and overall survival. (A) Relapse. The 2-year cumulative incidence of relapse was 42% (95% CI, 39-45) for Haplo relative donor (a) and 37% (95% CI, 30-45) for MUD (b) transplants. (B) Nonrelapse mortality. The 2-year cumulative incidence of nonrelapse mortality was 16% (95% CI, 14-19) for Haplo relative donor (a) and 8% (95% CI, 5-13) for MUD (b) transplants. (C) Overall survival. The 2-year probability of overall survival was 54% (95% CI, 51-57) for Haplo relative donor (a) and 67% (95% CI, 60-74) for MUD (b) transplants. (D) Disease-free survival. The 2-year probability of disease-free survival was 41% (95% CI, 38-45) for Haplo relative donor (a) and 55% (95% CI, 47-62) for MUD (b) transplants.
Reduced-intensity regimens: incidence of nonrelapse mortality, relapse, and disease-free and overall survival. (A) Relapse. The 2-year cumulative incidence of relapse was 42% (95% CI, 39-45) for Haplo relative donor (a) and 37% (95% CI, 30-45) for MUD (b) transplants. (B) Nonrelapse mortality. The 2-year cumulative incidence of nonrelapse mortality was 16% (95% CI, 14-19) for Haplo relative donor (a) and 8% (95% CI, 5-13) for MUD (b) transplants. (C) Overall survival. The 2-year probability of overall survival was 54% (95% CI, 51-57) for Haplo relative donor (a) and 67% (95% CI, 60-74) for MUD (b) transplants. (D) Disease-free survival. The 2-year probability of disease-free survival was 41% (95% CI, 38-45) for Haplo relative donor (a) and 55% (95% CI, 47-62) for MUD (b) transplants.
Causes of death
Five hundred sixty-eight of 1211 (47%) recipients of Haplo and 65 of 187 (35%) recipients of MUD HCT died. In both groups, disease recurrence was the most common cause of death, but the proportion was lower after Haplo HCT than with MUD HCT (55% vs 71%; P = .02). Among Haplo HCT recipients, 2% of deaths were due to graft failure, 7% to GVHD, 15% to infections, 3% to interstitial pneumonitis, 8% to organ failure, 2% to malignancy excluding primary diagnosis, and 5% to other causes; cause of death was not reported in 3% of recipients. Among MUD HCT recipients, 2% of deaths were due to GVHD, 6% to infections, 2% to interstitial pneumonitis, 14% to organ failure, and 3% to other causes; cause of death was not reported in 2% of recipients.
Myeloablative conditioning regimen HCT
Hematopoietic recovery
GVHD, relapse, nonrelapse mortality, and survival
Table 5 shows the risk of acute and chronic GVHD, nonrelapse mortality, relapse, and disease-free and overall survival by donor type. Compared with Haplo HCT, the risk of grades 3 and 4 but not 2 to 4 acute GVHD was lower with MUD HCT. Chronic GVHD risk was also lower with MUD HCT. The incidences of acute and chronic GVHD are shown in Table 3. Risks for nonrelapse mortality, relapse, and disease-free and overall survival did not differ by donor type and GVHD prophylaxis (Tables 3 and 5; Figure 2). A peripheral blood graft was associated with higher risk for chronic GVHD (P = .22), but the risk was independent of donor type. We examined for effect of transplant center by using the frailty model and found none. We also performed a sensitivity analysis limited to centers that performed both Haplo and MUD HCTs. Consistent with the main analysis, compared with Haplo HCT, we did not observe differences in survival after MUD HCT (HR, 0.82; 95% CI, 0.50-1.33; P = .41). Data on infections were available for a subset of patients (∼25% of study population). The 6-month incidence of bacterial infection was higher after Haplo HCT (56%; 95% CI, 51-61) than with MUD HCT (27%; 95% CI, 11-47; P = .003). There were no differences in the incidences of viral and fungal infections (data not shown). Eighty-two of 825 (10%) recipients of Haplo HCT received donor leukocyte infusion or underwent a second HCT compared with 6 of 79 (8%) recipients of MUD HCT (P = .28).
Myeloablative regimens: multivariate analysis of outcomes
| Outcome . | No. of events/evaluable . | HR (95% CI) . | P . |
|---|---|---|---|
| Grades 2 to 4 acute GVHD* | |||
| Haplo | 296/801 | 1.00 | |
| MUD | 32/95 | 0.92 (0.65-1.32) | .65 |
| Grades 3 and 4 acute GVHD* | |||
| Haplo | 87/798 | 1.00 | |
| MUD | 4/95 | 0.37 (0.14-1.00) | .050 |
| Chronic GVHD† | |||
| Haplo | 249/811 | 1.00 | |
| MUD, PTCy/CNI/MMF | 24/97 | 0.66 (0.43-1.01) | .053 |
| Relapse‡ | |||
| Haplo | 154/824 | 1.00 | |
| MUD | 19/97 | 1.03 (0.64-1.66) | .91 |
| Nonrelapse mortality§ | |||
| Haplo | 122/824 | 1.00 | |
| MUD | 14/97 | 0.77 (0.44-1.34) | .35 |
| Disease-free survival║ | |||
| Haplo | 276/824 | 1.00 | |
| MUD | 33/97 | 0.89 (0.62-1.38) | .53 |
| Overall survival¶ | |||
| Haplo | 202/824 | 1.00 | |
| MUD | 21/97 | 0.70 (0.44-1.09) | .12 |
| Outcome . | No. of events/evaluable . | HR (95% CI) . | P . |
|---|---|---|---|
| Grades 2 to 4 acute GVHD* | |||
| Haplo | 296/801 | 1.00 | |
| MUD | 32/95 | 0.92 (0.65-1.32) | .65 |
| Grades 3 and 4 acute GVHD* | |||
| Haplo | 87/798 | 1.00 | |
| MUD | 4/95 | 0.37 (0.14-1.00) | .050 |
| Chronic GVHD† | |||
| Haplo | 249/811 | 1.00 | |
| MUD, PTCy/CNI/MMF | 24/97 | 0.66 (0.43-1.01) | .053 |
| Relapse‡ | |||
| Haplo | 154/824 | 1.00 | |
| MUD | 19/97 | 1.03 (0.64-1.66) | .91 |
| Nonrelapse mortality§ | |||
| Haplo | 122/824 | 1.00 | |
| MUD | 14/97 | 0.77 (0.44-1.34) | .35 |
| Disease-free survival║ | |||
| Haplo | 276/824 | 1.00 | |
| MUD | 33/97 | 0.89 (0.62-1.38) | .53 |
| Overall survival¶ | |||
| Haplo | 202/824 | 1.00 | |
| MUD | 21/97 | 0.70 (0.44-1.09) | .12 |
Model unadjusted, no significant factors.
Model adjusted for graft type (peripheral blood: HR, 2.06; 95% CI, 1.48-2.87; P < .0001).
Model adjusted for disease risk index (high-risk: HR, 1.80; 95% CI, 1.28-2.53; P = .0007) and conditioning regimen (non-TBI: HR, 1.59; 95% CI, 1.17-2.16; P = .003).
Model adjusted for age (>55 y: HR, 2.76; 95% CI, 1.95-3.92; P < .0001), hematopoietic comorbidity index (≥3: HR, 1.53; 95% CI, 1.08-2.15, P = .015), and conditioning regimen (non-TBI: HR, 1.46; 95% CI, 1.01-2.09; P = .04).
Model adjusted for age (>55 y: HR, 1.62; 95% CI, 1.28-2.05; P < .0001), hematopoietic comorbidity index (≥3: HR, 1.37; 95% CI, 1.09-1.72; P = .006), disease risk index (high-risk: HR, 1.66; 95% CI, 1.29-2.14; P = .0001), and conditioning regimen (non-TBI: HR, 1.55; 95% CI, 1.22-1.97; P = .003).
Model adjusted for age (>55 y: HR, 2.03; 95% CI, 1.54-2.67; P < .0001), hematopoietic comorbidity index (≥3: HR, 1.40; 95% CI, 1.07-1.82; P = .02), disease risk index (high-risk: HR, 1.60; 95% CI, 1.19-2.16; P = .002), and conditioning regimen (non-TBI: HR, 1.65; 95% CI, 1.24-2.19; P = .0006).
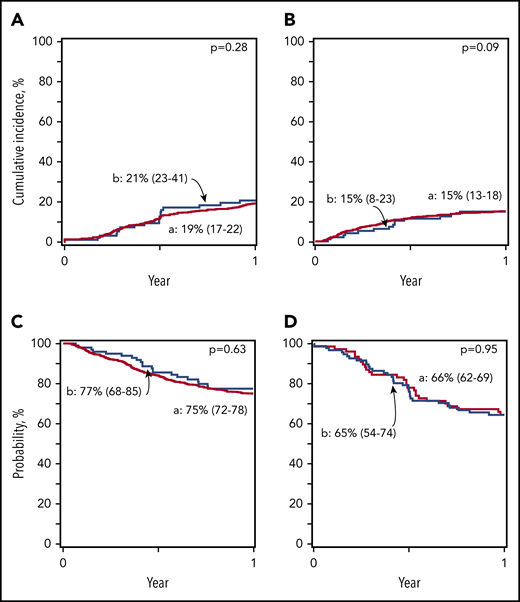
Myeloablative regimens: incidence of nonrelapse mortality, relapse, and disease-free and overall survival. (A) Relapse. The 1-year cumulative incidence of relapse was 19% (95% CI, 17-22) for Haplo relative donor (a) and 21% (95% CI, 23-41) for MUD (b) transplants. (B) Nonrelapse mortality. The 1-year cumulative incidence of nonrelapse mortality was 15% (95% CI, 13-18) for Haplo relative donor (a) and 15% (95% CI, 8-23) for MUD (b) transplants. (C) Overall survival. The 1-year probability of overall survival was 75% (95% CI, 72-78) for Haplo relative donor (a) and 77% (95% CI, 68-85) for MUD (b) transplants. (D) Disease-free survival. The 1-year probability of disease-free survival was 66% (95% CI, 62-69) for Haplo relative donor (a) and 65% (95% CI, 54-74) for MUD (b) transplants.
Myeloablative regimens: incidence of nonrelapse mortality, relapse, and disease-free and overall survival. (A) Relapse. The 1-year cumulative incidence of relapse was 19% (95% CI, 17-22) for Haplo relative donor (a) and 21% (95% CI, 23-41) for MUD (b) transplants. (B) Nonrelapse mortality. The 1-year cumulative incidence of nonrelapse mortality was 15% (95% CI, 13-18) for Haplo relative donor (a) and 15% (95% CI, 8-23) for MUD (b) transplants. (C) Overall survival. The 1-year probability of overall survival was 75% (95% CI, 72-78) for Haplo relative donor (a) and 77% (95% CI, 68-85) for MUD (b) transplants. (D) Disease-free survival. The 1-year probability of disease-free survival was 66% (95% CI, 62-69) for Haplo relative donor (a) and 65% (95% CI, 54-74) for MUD (b) transplants.
Causes of death
Two hundred seventy-five of 825 (33%) recipients of Haplo HCT and 48 of 176 (27%) recipients of MUD HCT died. There were no significant differences in causes of death between the groups (P = .96). Recurrent disease was the most common cause of death in both groups, (50% vs 47%). Among Haplo HCT recipients, 3% of deaths were due to graft failure, 9% to GVHD, 12% to infections, 7% to interstitial pneumonitis, 11% to organ failure, and 7% to other causes; cause of death was not reported in 3% of the recipients. Among the MUD HCT recipients, 2% of deaths were due to graft failure, 10% to GVHD, 10% to infections, 2% to interstitial pneumonitis, 13% to organ failure, and 8% to other causes; cause of death was not reported in 4% of recipients.
Subset analysis
We acknowledge that there are differences in characteristics between donor types and that most MUD HCTs occurred in a more recent period. Therefore, a subset analysis was conducted, limited to the period 2016 through 2018, Whites, and recipients of peripheral blood grafts. Consistent with the main analysis, after reduced-intensity conditioning, nonrelapse mortality risks (HR, 0.34; 95% CI, 0.15-0.73; P = .006) were lower after MUD HCT resulting in higher disease-free (HR, 0.69; 95% CI, 0.50-0.94; P = .018) and overall (HR, 0.57; 95% CI, 0.39-0.84; P = .004) survival than with Haplo HCT. Also consistent with the main analysis, after myeloablative conditioning, nonrelapse mortality risks (HR, 0.74; 95% CI, 0.36-1.52; P = .41), and disease-free (HR, 1.02; 95% CI, 0.64-1.61; P = .95) and overall (HR, 0.62; 95% CI; 0.34-1.15; P = .13) survival did not differ by donor type. Relapse risks did not differ by donor type with reduced-intensity and myeloablative conditioning regimens (data not shown). Grades 3 and 4 acute GVHD risks were lower after MUD HCT with the reduced-intensity (HR, 0.38; 95% CI, 0.15-0.97; P = .04) and myeloablative (HR, 0.33; 95% CI, 0.10-1.07; P = .06) regimens.
Discussion
An earlier comparison of outcomes of Haplo and MUD HCTs did not show differences in nonrelapse mortality, relapse, or disease-free and overall survival, but conditioning regimen intensity, graft type, and GVHD prophylaxis for the donor types differed.10 To overcome the limitations stated above, the current analyses adopted the following approach: (1) reduced-intensity and myeloablative conditioning regimen HCTs were analyzed separately to account for potential differences in nonrelapse mortality and relapse risks; (2) peripheral blood was the predominant graft that was used for both donor types, and subset analyses were performed that were limited to peripheral blood grafts in a recent period; and (3) both Haplo and MUD HCT recipients received PTCy/CNI/mycophenolate for GVHD prophylaxis. After a controlled analysis that adjusted for patient, disease, and transplant characteristics, the data favored MUD HCT. Among the recipients of reduced-intensity conditioning, the incidences of graft failure, grades 3 and 4 acute GVHD, and nonrelapse mortality were higher after Haplo HCT. Consequently, disease-free and overall survival were lower after Haplo HCT than after MUD HCT. Among the recipients of myeloablative conditioning, the incidences of grades 3 and 4 acute GVHD were higher after Haplo HCT, but there were no differences in nonrelapse mortality or disease-free or overall survival between Haplo and MUD HCT. Successful management of severe acute GVHD is challenging and adds to the burden of morbidity and mortality in the long term. The adoption of PTCy-containing GVHD prophylaxis for myeloablative MUD HCTs is relatively recent and our patients had a median follow-up of only 12 months. Nevertheless, lower survival after reduced-intensity Haplo HCT and a higher incidence of severe acute GVHD after myeloablative HCT confirmed that donor-recipient HLA matching is an important determinant of outcomes after transplantation.
Compared with historical cohorts of MUD HCTs with CNI/methotrexate or mycophenolate GVHD prophylaxis, there was a 20% to 25% reduction in the incidence of chronic GVHD with PTCy as the backbone of the GVHD prophylaxis.20,21 We hypothesize that the GVHD prophylaxis of PTCy/CNI/mycophenolate for MUD HCT blunted the expected higher GVHD risk typically seen with CNI/methotrexate or mycophenolate GVHD prophylaxis. Consistent with published reports, transplantation of peripheral blood from either donor type was associated with higher incidence of chronic GVHD.22,23 We did not assess the functional health of surviving patients, which may be affected by a higher incidence of chronic GVHD after transplantation of peripheral blood.24 We are limited in our ability to study the effect of severe acute and chronic GVHD on long-term survival, given the modest follow-up of our cohort.
The observed higher nonrelapse and overall mortality after reduced-intensity conditioning Haplo HCT can be attributed to the higher incidence of graft failure and grades 3 and 4 acute GVHD. An examination of graft failure rates in recipients of Haplo HCT with reduced-intensity regimens did not detect differences between bone marrow (10%; 95% CI, 7-14) and peripheral blood (11%; 95% CI, 8-14; P = .92). We did not observe differences in incidence of viral infections after Haplo and MUD HCT, regardless of conditioning regimen intensity, although some reports suggest that higher cytomegalovirus reactivation is associated with Haplo HCT.25 Data on infections were available for a subset of patients; those observations must be validated in a larger population.
Ideally, studies aimed at identifying an optimal donor for HCT should be randomized trials, but the logistical considerations are formidable. For example, in a randomized trial, all potentially eligible subjects would ideally have both a suitable Haplo donor and MUD before randomization. Such a criterion would limit inclusion of non-White subjects who are less likely to have available MUDs, would lengthen accrual time and costs associated with trial management, and would exclude patients with high-risk disease who are likely to benefit from an expeditious HCT. We acknowledge the limitation that our findings were derived from data reported to a large transplant registry. Expertise with a donor type or donor selection algorithms influence donor selection at the transplant center. However, to our knowledge, this is the first report in which both Haplo and MUD HCT recipients received uniform GVHD prophylaxis regimens and were studied separately based on the intensity of the transplant conditioning regimen. Regardless of regimen intensity, we did not observe differences in relapse by donor type. The higher incidence of graft failure, severe acute GVHD, and mortality after reduced-intensity conditioning Haplo HCT and higher severe acute GVHD after myeloablative Haplo HCT favor a MUD, if such a donor is readily available. Access to transplantation extends survival and patients who are likely to benefit from HCT should be offered Haplo HCT if a MUD is not readily available.
The study data set is available upon request at https://www.cibmtr.org/referencecenter/publist/pubdsdownload/pages/default.aspx.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
There is a Blood Commentary on this article in this issue.
Acknowledgments
This work was supported primarily by National Institutes of Health (NIH)/National Cancer Institute (NCI) grant U24-CA076518, the NIH/National Heart, Lung and Blood Institute (NHLBI), the NIH/National Institute of Allergy and Infectious Diseases (NIAID), and contract HHSH250201200016C from the Health Resources and Services Administration/Department of Health and Human Services (HRSA/DHHS). The views expressed in this article do not reflect the official policy or position of the NIH, HRSA, or any other agency of the US Government.
Authorship
Contribution: M.G., R. Romee, A.S., and M.E. designed the study; A.S. analyzed the data; M.G., R. Romee, A.S., R.J.S., and M.E. interpreted the data; M.G. drafted the manuscript; R. Romee, A.S.M., M.A., M.A.M., J.H.A., C.N.B., C.G.B., S.C., E.J.F., N.G., M.R.G., C.G.K., N.K., J.P.M., I.K.M., R.S.M., M.M., F.M., D.M., R. Reshef, S.R.S., M.A.S., E.K.W., Y.I., R.J.S., and M.E. reviewed and interpreted the data, and critically reviewed the manuscript; and all authors approved the final version of the manuscript.
Conflict-of-interest disclosure: M.R.G. has received consulting fees from AbbVie, Agios, Amgen, Astellas, Cardinal Health, Bristol-Myers Squibb/Celgene, Daiichi Sankyo, Gilead, Incyte, Karius, Merck, Pfizer, Premier, Stemline, and Trovagene and research funding from Forma Therapeutics, Genentech/Roche, Incyte, and Janssen. R.J.S. serves on the Board of Directors for Kiadis and Be The Match/National Marrow Donor Program; has provided consulting for Gilead, Rheos Therapeutics, VOR Biopharma, and Novartis; and has served on the Data Safety Monitoring Board for Juno, Celgene, and BMS. The remaining authors declare no competing financial interests.
Correspondence: Mahasweta Gooptu, Department of Hematology/Oncology, Dana-Farber Cancer Institute, Boston MA 02115; e-mail: mahasweta_gooptu@dfci.harvard.edu.
REFERENCES
Author notes
M.G. and R.R. contributed equally to this study.

Comments
The Impact of Donor Type on Outcomes is Clear as MUD
HaploD age is tightly correlated with recipient-age by virtue of donor and recipient relatedness (see figure 1 in Dezern et al’s publication);3 however, there is no such association between recipient and MUD ages. In practices which de-emphasize donor age, haploD age, but not MUD age, increases as recipient-age increases. The confounding will then manifest in comparisons of donor type for older recipients, but not for younger ones. Gooptu et al stratified their analysis by conditioning intensity, and indirectly, age. The comparison in the older RIC group may therefore be confounded by large differences in donor-age of haploD and MUD, explaining why differences in BMT outcomes were greater with RIC than MAC. Conversely, it is not clear why HLA-mismatch should have a greater effect on BMT outcomes with RIC than MAC.
To avoid the potentially erroneous conclusion that HLA-mismatch is to blame for poorer outcomes with RIC, the role of donor-age must be clarified. After all, older age is not an inherent feature of haplo-BMT. The recipient-HaploD age correlation can be disrupted by purposefully selecting younger family donors. If our hypothesis is correct, then a potential haploD grandchild should not be eschewed in favor of a MUD, especially at the expense of timeliness.
References:
1. Gooptu M, Romee R, St. Martin A, et al. HLA Haploidentical versus Matched Unrelated Donor Transplants with Post-Transplant Cyclophosphamide based prophylaxis. [published online ahead of print 13 April 2021]. Blood. doi: https://doi.org/10.1182/blood.2021011281.
2. Kollman C, Spellman SR, Zhang MJ, et al. The effect of donor characteristics on survival after unrelated donor transplantation for hematologic malignancy. Blood. 2016;127(2):260–267.
3. DeZern AE, Franklin C, Tsai H-L, et al. Relationship of donor age and relationship to outcomes of haploidentical transplantation with posttransplant cyclophosphamide. Blood Adv. 2021;5(5):1360–1368.