Key Points
TNF and IL-6 are produced during acute graft-versus-host disease (GVHD).
Combined blockade of both TNF and IL-6R results in greater protection from GVHD and does not impair graft-versus-tumor (GVT) effects.
Abstract
Allogeneic hematopoietic stem cell transplantation (allo-HSCT) remains a potential curative option for treating a variety of hematologic diseases, but acute and chronic graft-versus-host disease (GVHD) remain major barriers limiting efficacy. Acute gut GVHD occurs with marked increases in proinflammatory cytokines (including TNF and IL-6), which we recently demonstrated was exacerbated in obesity resulting in severe gastrointestinal pathology. Given the pleiotropic and overlapping effects of these 2 cytokines, we assessed the impact of dual TNF and IL-6R blockade on GVHD as well as graft-versus tumor (GVT) effects in different mouse GVHD models. Early administration of combined blockade resulted in greater protection and survival from acute gut GVHD compared with single blockade regimens and even development of later chronic skin GVHD. Importantly, double cytokine blockade preserved GVT effects reinforcing that GVT and GVHD can be delineated and may result in greater efficacy in allo-HSCT.
Introduction
Acute GVHD (aGVHD) from allo-HSCT represents an inflammation-driven disease characterized by rapid and lethal multi-organ damage, whereas chronic GVHD (cGVHD) can be delayed and characterized by tissue fibrosis.1 A primary advantage of allo-HSCT is the graft-versus-tumor (GVT) effects.2 Extensive studies have focused on suppressing GVHD pathogenesis while maintaining the GVT effects; nevertheless, GVHD still dominates the clinical picture limiting efficacy.1,2
Acute GVHD is associated with marked increases in pro-inflammatory cytokines caused by “cytokine storm,”3 similar to the paradigm that occurs after applying strong systemic cancer immunotherapies in obese or aged mice.4-6 We recently observed that obesity exacerbated lethal acute gastrointestinal GVHD with similar increases of pro-inflammatory cytokines.7
Similar with autoimmune diseases, blockade of IL-6 and TNF has been under extensive investigation in allo-HSCT with focus on single cytokine blockade showing variable and contradicting results.8-10 Blocking of IL-6 signaling results in significant reduction but not complete protection against GVHD.11-14 Surprisingly, given the complementary and pleiotropic actions of these two cytokines, the efficacy of dual of IL-6 and TNF blockade remained untested. We therefore examined the effect of cytokine blockade on outcomes following allo-HSCT on both GVHD and GVT in different models demonstrating superior efficacy.
Study design
Detailed methods are provided in the supplemental Materials, available on the Blood Web site.
Mice and allo-HSCT
Seven- to 8-week-old BALB/c and C57BL/6 female mice used in this study were obtained from Taconic Biosciences (Germantown, NY). BALB/c mice were placed on a high-fat diet (60% fat from lard, D12492) or low-fat diet (10% fat from lard, D12450J) from Research Diets, Inc for 4-6 months to create DIO (>40 g) and control mice (22-28 g). 8- to 12-week-old B10.D2 and BALB/c mice were obtained from The Jackson Laboratory (Bar Harbor, ME) and used as donor mice. To create a minor mismatch GVHD model, control and DIO BALB/c mice (H2d) received lethal total body irradiation (TBI, single-dose 800 cGy; 137Cs source) and underwent transplantation with 8 × 106 bone marrow (BM) cells with or without splenocytes (SC,10-25 × 106) from donor B10.D2 mice (H2d), as described previously.7,15
To create a MHC-mismatched GVHD model, C57BL/6 mice (H2b) received lethal TBI (single-dose 1050 cGy; 137Cs source) and 5 × 106 BM cells with or without SCs (40 × 106) from donor BALB/c mice (H2d). For GVT experiments using this model, 5 × 106 C1498 (acute myeloid leukemia) cells tagged with Cell Trace Violet (catalog no. 34557; ThermoFisher) were given IV.
All data were obtained from at ≥2 independent experiments with similar results. All mice were maintained at the University of California, Davis Medical Center's vivarium in accordance with Institutional Animal Care and Use Committee (IACUC) standards.
Bioluminescent imaging
The A20 (B-cell lymphoma) cell line transfected with luciferase was injected IV into recipient BALB/c mice at a dose of 1 × 106 cells on the same day of allo-HSCT. Tumor growth was monitored with an IVIS Spectrum In Vivo Imaging System (PerkinElmer, Waltham, MA). In brief, mice were anesthetized with isoflurane, injected with d-luciferin (3 mg/mouse, intraperitoneally [i.p.]), then imaged 5 minutes postinjection with the IVIS Spectrum system. Bioluminescence data were acquired using Living Image 3.0 (Caliper Life Sciences, Hopkinton, MA) and analyzed by GraphPad Prism (GraphPad Software).
Reagents
Anti-mouse IL-6R monoclonal antibody (clone 15A7; BioXCell) and Enbrel (etanercept) were used in the single or combination therapy experiments. Mice received 0.5 mg of anti–IL-6R i.p. and 1.5 mg of etanercept subcutaneously (s.c.) one day before HSCT and every 3 days after until day 8 post–allo-HSCT. Rat and human IgG controls were purchased from Jackson ImmunoResearch Laboratories) and administered at the same doses and injection routes of anti-IL-6R and etanercept, respectively.
Results and discussion
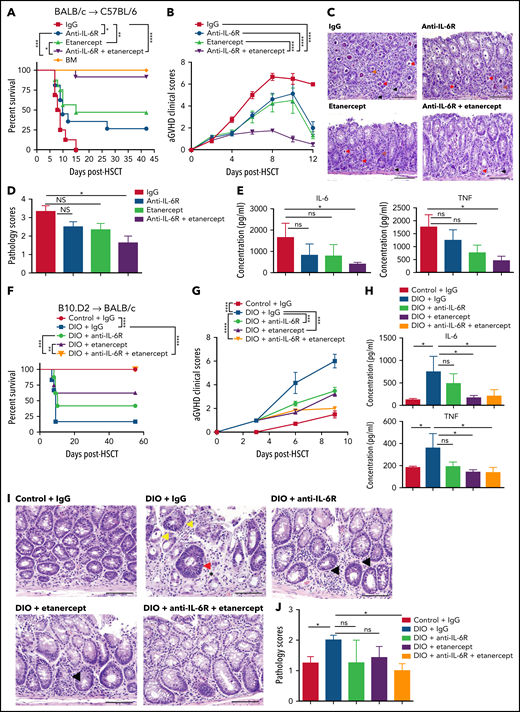
Based on previous observations that cytokine storm-driven lethality in obese mice after systemic immunotherapy or LPS could only partially be abrogated by TNF blockade4 and reports by others that single cytokine blockade of TNF or IL-6R could also offer partial protection,10-12 we hypothesized that dual blockade of these two cytokines may offer greater protection. Using an MHC mismatch model, the combination of cytokine blockade exhibited superior protective effects against aGVHD severity and mortality compared with single blockade (Figure 1A-B). Combined blockade also ameliorated the GVHD pathogenesis targeting the gastrointestinal (GI) tract (Figure 1C-D) and limited GI tract inflammation (supplemental Figure 1A ). In line with that observation, we observed that the levels of IL-6 and TNF in the serum (Figure 1E), as well as endotoxin translocation originating from the damaged GI tract (supplemental Figure 1B) were significantly reduced with the administration of dual cytokine blockade compared with single blockade.
Effects of TNF and IL6 blockade on acute GVHD after alloHSCT in mice. (A-E) Lethally irradiated C57BL/6 mice received 5 × 106 bone marrow (BM) cells with and without 40 × 106 spleen cells (SCs) from donor BALB/c mice and were treated with either single or dual cytokine blockade. (A) Survival rate (BM: n = 4, other groups: n = 16 per group). (B) aGVHD clinical scores (n = 8 per group). (C) Representative images of H&E staining from colon samples at day 9 post-HSCT. Red arrows indicate crypt apoptosis, black arrows indicate lamina propial lymphocytes, orange arrows indicate intracryptal lymphocytes. The scale bar, 100 μm. (D) Pathology scores of GI tract samples (n = 5-7 per group). (E) Serum IL-6 and serum TNF concentrations at day 6 post-HSCT (n = 5-7 per group). (F-J) Lethally irradiated control and DIO BALB/c mice received 8 × 106 BM cells and 25 × 106 SCs from donor B10.D2 mice and were treated with either single or dual cytokine blockade. (F) Survival rate (n = 7-8 per group) (G) aGVHD clinical scores at day 6 post-HSCT (n = 7-8 per group). (H) Serum IL-6 and TNF concentrations at day 7 post-HSCT (n = 5-7 per group). (I) Representative images of H&E staining from colon samples at day 7 post-HSCT. The scale bar is 100 μm. Arrows indicate crypt apoptosis. Much milder lamina proprial lymphocytic infiltration is noted with dual cytokine blockade compared with single blockade. (J) Pathology scores of samples from panel H (n = 5-6 per group). Bar graphs depict mean ± SEM. Survival curves (A,F) were plotted on a Kaplan-Meier curve and analyzed by a log-rank test. Clinical scores were analyzed by 2-way analysis of variance (ANOVA) with Tukey's post hoc test for comparison among groups. A 1-way ANOVA test was used in panels E, D, H, and J. *P < .05; **P < .01; ***P < .001; ****P < .0001.
Effects of TNF and IL6 blockade on acute GVHD after alloHSCT in mice. (A-E) Lethally irradiated C57BL/6 mice received 5 × 106 bone marrow (BM) cells with and without 40 × 106 spleen cells (SCs) from donor BALB/c mice and were treated with either single or dual cytokine blockade. (A) Survival rate (BM: n = 4, other groups: n = 16 per group). (B) aGVHD clinical scores (n = 8 per group). (C) Representative images of H&E staining from colon samples at day 9 post-HSCT. Red arrows indicate crypt apoptosis, black arrows indicate lamina propial lymphocytes, orange arrows indicate intracryptal lymphocytes. The scale bar, 100 μm. (D) Pathology scores of GI tract samples (n = 5-7 per group). (E) Serum IL-6 and serum TNF concentrations at day 6 post-HSCT (n = 5-7 per group). (F-J) Lethally irradiated control and DIO BALB/c mice received 8 × 106 BM cells and 25 × 106 SCs from donor B10.D2 mice and were treated with either single or dual cytokine blockade. (F) Survival rate (n = 7-8 per group) (G) aGVHD clinical scores at day 6 post-HSCT (n = 7-8 per group). (H) Serum IL-6 and TNF concentrations at day 7 post-HSCT (n = 5-7 per group). (I) Representative images of H&E staining from colon samples at day 7 post-HSCT. The scale bar is 100 μm. Arrows indicate crypt apoptosis. Much milder lamina proprial lymphocytic infiltration is noted with dual cytokine blockade compared with single blockade. (J) Pathology scores of samples from panel H (n = 5-6 per group). Bar graphs depict mean ± SEM. Survival curves (A,F) were plotted on a Kaplan-Meier curve and analyzed by a log-rank test. Clinical scores were analyzed by 2-way analysis of variance (ANOVA) with Tukey's post hoc test for comparison among groups. A 1-way ANOVA test was used in panels E, D, H, and J. *P < .05; **P < .01; ***P < .001; ****P < .0001.
We then assessed the efficacy of dual blockade in the setting of obesity (supplemental Figure 1C) using a minor mismatch model given the rapid onset of acute lethal gut GVHD using diet-induced obese (DIO) recipients.7 Treatment of DIO recipients with dual cytokine blockade resulted in significant protection against accelerated aGVHD compared with untreated DIO mice (Figure 1F-G) with a significant decrease of serum levels of IL-6 and TNF (Figure 1H). Gross morphology demonstrated the greatest protection of dual blockade on GI tract diarrhea and inflammation (supplemental Figure 2A,B ). Histology assessment by H&E staining also showed that dual blockade maintained the structure of the GI tract with less lymphocyte infiltration and less crypt loss compared with untreated or single blockade groups (Figure 1I-J). Serum endotoxin levels were significantly reduced following the dual blockade treatment, which was not observed after single cytokine blockade (supplemental Figure 2C). Interestingly, in our study, gene expression data analysis also revealed that dual cytokine blockade treatment maintained the expression of IL-22, a cytokine that promotes intestinal regeneration and regulates DNA damage response in intestinal stem cells,16 and supported the induction of regulatory T cells (Tregs)11,17 (supplemental Figure 2D). Neither single blockade nor dual blockade impaired donor cell engraftment (supplemental Figures 3-4 ).
Because in a minor mismatch model, the outcome in DIO recipients shifts from a typical skin cGVHD to the lethal gut aGVHD,7 we assessed effects on cGVHD at later time points as the DIO recipients receiving the dual blockade now survived. DIO recipients of dual cytokine blockade treatment did develop sclerodermatous cGVHD, albeit milder, at later timepoints compared with control mice (supplemental Figure 5A-C). Interestingly, we did not observe comparable skin alopecia compared with the lean counterparts and the DIO recipients had significantly reduced cGVHD symptoms (supplemental Figure 5D). The cGVHD developed in the DIO and control recipients were both still characterized by collagen deposition and fat tissue atrophy revealed by Trichrome staining (supplemental Figure 5E). In studies using young lean BALB/c mice, we observed that either single or dual blockade had similar protective effects on sclerodermatous GVHD symptoms and while there was not a significant difference between single and combination blockade groups, more research is still needed with cGVHD models to definitively determine whether additive protection can be achieved (supplemental Figure 6A-B). Taken together, dual blocking IL-6 and TNF pathways primarily shows increased efficacy in acute GVHD protection but also partially protected mice from cGVHD in the skin long after cessation of treatment.
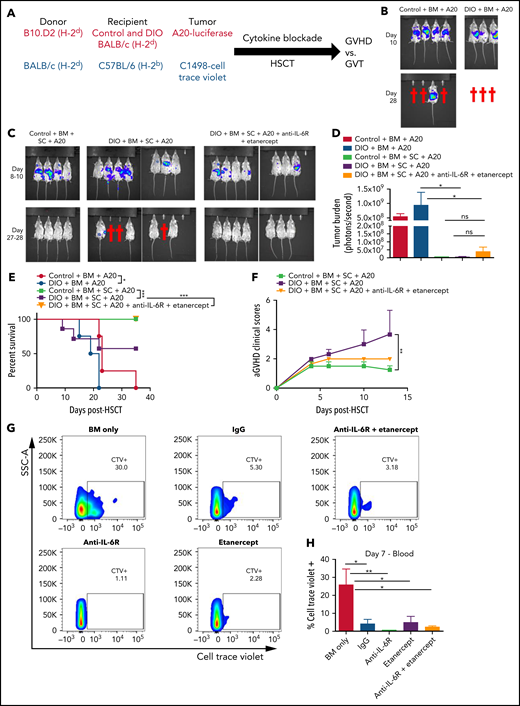
We then assessed whether this dual cytokine blockade would impact beneficial GVT effects. To address this, we used two different allo-HSCT tumor models, first DIO and control BALB/c mice were given A20 (B-cell lymphoma) cells and in the second, C57BL/6 mice given C1498 (acute myeloid leukemia) cells (Figure 2A). Lymphoma growth was markedly accelerated in DIO mice (Figure 2B,E ), which has been previously reported by our group and others.18,19 Following allo-HSCT, DIO recipients succumbed to aGVHD, however, dual cytokine blockade resulted in protection from GVHD lethality and pathology while maintaining GVT effects as determined by loss of tumor burden in the treated groups and clearance after 4 weeks (Figure 2C-F). Similar results were obtained using the C1498 model with cytokine blockade preserving GVT capability (Figure 2G-H). These results demonstrate that combined pro-inflammatory cytokine blockade targeting IL-6R and TNF rescues recipients from the cytokine storm and lethal gut GVHD while still preserving GVT processes.
Effects of TNF and IL6 blockade on graft-versus-tumor effects after alloHSCT in mice. (A) Schema of two GVT models. Lethally irradiated control and DIO BALB/c mice received 8 × 106 BM cells and 10 × 106 SCs from donor B10.D2 mice, 1 × 106 A20-luciferase cells, and were treated with dual cytokine blockade (B-F). Lethally irradiated young lean C57BL/6 mice received 5 × 106 BM cells and 20 × 106 SCs from donor BALB/c mice, 5 × 106 Cell Trace Violet-tagged C1498 cells, and were treated with single and dual cytokine blockade (G-H). (B) Representative images of tumor burden at day 10 and day 28 post-HSCT, and tumor injection of control and DIO mice transplanted with BM cells and A20 cells. (C) Representative images of tumor burden at day 8 to day 28 post-HSCT and tumor injection of recipients with bone marrow cells and splenocytes from B10.D2 mice, A20 cells, with or without cytokine blockade treatment. (D) Quantification of A20 burden at day 10 post-HSCT and tumor injection (n = 4-8 per group). (E) Survival rate (n = 4-8 per group). (F) aGVHD clinical scores (n = 7-8 per group). (G) Representative flow cytometry plots of percentages of C1498 cells tagged with Cell Trace Violet in the peripheral blood at day 7 post-HSCT. (H) Quantification of percentages of C1498 cells (n = 4 per group). Bar graphs depict mean ± SEM. Survival curves were plotted on a Kaplan-Meier curve and analyzed by a log-rank test. One-way ANOVA test was used in panel D. Two-way ANOVA test was used in panel F. *P < .05; **P < .01; ***P < .001; ns, not significant.
Effects of TNF and IL6 blockade on graft-versus-tumor effects after alloHSCT in mice. (A) Schema of two GVT models. Lethally irradiated control and DIO BALB/c mice received 8 × 106 BM cells and 10 × 106 SCs from donor B10.D2 mice, 1 × 106 A20-luciferase cells, and were treated with dual cytokine blockade (B-F). Lethally irradiated young lean C57BL/6 mice received 5 × 106 BM cells and 20 × 106 SCs from donor BALB/c mice, 5 × 106 Cell Trace Violet-tagged C1498 cells, and were treated with single and dual cytokine blockade (G-H). (B) Representative images of tumor burden at day 10 and day 28 post-HSCT, and tumor injection of control and DIO mice transplanted with BM cells and A20 cells. (C) Representative images of tumor burden at day 8 to day 28 post-HSCT and tumor injection of recipients with bone marrow cells and splenocytes from B10.D2 mice, A20 cells, with or without cytokine blockade treatment. (D) Quantification of A20 burden at day 10 post-HSCT and tumor injection (n = 4-8 per group). (E) Survival rate (n = 4-8 per group). (F) aGVHD clinical scores (n = 7-8 per group). (G) Representative flow cytometry plots of percentages of C1498 cells tagged with Cell Trace Violet in the peripheral blood at day 7 post-HSCT. (H) Quantification of percentages of C1498 cells (n = 4 per group). Bar graphs depict mean ± SEM. Survival curves were plotted on a Kaplan-Meier curve and analyzed by a log-rank test. One-way ANOVA test was used in panel D. Two-way ANOVA test was used in panel F. *P < .05; **P < .01; ***P < .001; ns, not significant.
Targeting proinflammatory cytokines such as TNF and IL-6 has been studied as single agent modalities in HSCT but not in combination.8-14,20 We demonstrated that dual blockade of IL-6R and TNF signaling resulted in significantly greater protection in either MHC mismatch or minor mismatch models impacting gut aGVHD and even later sclerodermatous cGVHD. Despite having promising data, our study still has limitations in that it lacks an extensive characterization of dual cytokine blockade effect in other GVHD target tissues and for the treatment of ongoing GVHD. Interestingly, not just aGVHD was impacted as surviving mice still developed chronic skin GVHD but to a lesser extent even though cytokine blockade ended after the first week suggesting the altering of GVHD outcomes in the obese models to aGVHD also impacted later cGVHD pathology. While IL-6 and TNF have multiple and different effects on different organs and cell types, the consistent upregulation of both in highly inflammatory states suggests that targeting both would allow for greater suppression of inflammatory processes, possibly impacting more tissues where differential effects could be attributed to either one. These results may add context to the disappointing clinical results of TNF blockade on GVHD.21,22 Using proinflammatory cytokines previously documented to impact acute GVHD in combination suggests compensation mechanisms may need to be counteracted for greater efficacy. However, there may also be species differences and nuances in the models used, which rely on a particular cytokine or GVHD pathway, and needs to be taken into consideration before extrapolating to humans.
It will be important to more completely assess effects of combination blockade on GVT effects as well as tumor growth in general because IL-6 has been demonstrated to be pro-tumorigenic in many cancers23,24 and blockade may offer additional GVT effects.
In a broader perspective, cytokine storm or cytokine release syndrome is a phenomenon that occurs in various immunopathogenic situations such as with chimeric antigen receptor T-cell application, sepsis or after acute viral infection such as COVID-19.25 The data presented here demonstrating increased efficacy of combined blockade suggests that combined cytokine blockade in treating inflammation-mediated pathology may have greater benefit in other clinical settings.
Acknowledgments
The authors thank Sean Judge and Hyeongsun Moon for their expertise and insightful feedback and suggestions. They also thank Weihong Ma from the W.J.M. laboratory, and Qian Chen in the UC Davis Pathology Core for their technical expertise and help.
This work was funded by National Institutes of Health (NIH), National Cancer Institute R01 CA214048 (W.J.M.), NIH, National Institute of Allergy and Infectious Diseases R37 AI34495, NIH, National Heart, Lung, and Blood Institute HL56067 (B.R.B.) and the UC Davis Comprehensive Cancer Center Support Grant (CCSG) (P30 CA093373). M.D. is supported by NIH, National Institute of Diabetes and Digestive and Kidney Diseases K08DK110421.
The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products or organizations imply endorsement by the US Government.
Authorship
Contribution: L.T.K. designed and performed experiments, analyzed results, and cowrote the manuscript; L.V.V., C.P.C., C.D., S.K.M., C.T.L., C.-C.S.P., and K.M.S. performed experiments and edited the manuscript; E.C. performed histology scores and edited the manuscript; E.M., R.J.C., A.M.M., D.L.L., M.A., B.R.B., and M.D. edited the manuscript; and W.J.M. directed the project, designed experiments, interpreted results, and cowrote the manuscript.
Conflict-of-interest disclosure: M.A. is a member of advisory board for Kite and speaker bureau for Gilead, BMS, Abbvie, Kite, and Celgene. B.R.B. receives remuneration as an advisor to Magenta Therapeutics and BlueRock Therapeutics; research funding from BlueRock Therapeutics, Rheos Medicines, Equilibre biopharmaceuticals, Carisma Therapeutics, Inc., and is a cofounder of Tmunity Therapeutics. A.M.M. has advisory role or research funding from Merck, Genentech, BMS, Incyte, Trisalus, MultiplexThera, EMD serono, and Transgene.
Correspondence: William J. Murphy, Department of Dermatology and Department of Internal Medicine, School of Medicine, University of California, Davis, Sacramento, CA 95817; e-mail: wmjmurphy@ucdavis.edu.
The online version of this article contains a data supplement.
There is a Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal