Abstract
Congenital dysfibrinogenemia (CD) is caused by structural changes in fibrinogen that modify its function. Diagnosis is based on discrepancy between decreased fibrinogen activity and normal fibrinogen antigen levels and is confirmed by genetic testing. CD is caused by monoallelic mutations in fibrinogen genes that lead to clinically heterogenous disorders. Most patients with CD are asymptomatic at the time of diagnosis, but the clinical course may be complicated by a tendency toward bleeding and/or thrombosis. Patients with a thrombosis-related fibrinogen variant are particularly at risk, and, in such patients, long-term anticoagulation should be considered. Management of surgery and pregnancy raise important and difficult issues. The mainstay of CD treatment remains fibrinogen supplementation. Antifibrinolytic agents are part of the treatment in some specific clinical settings. In this article, we discuss 5 clinical scenarios to highlight common clinical challenges. We detail our approach to establishing a diagnosis of CD and discuss strategies for the management of bleeding, thrombosis, surgery, and pregnancy.
Introduction
Fibrinogen plays a central role in hemostasis as a support for platelet aggregation, a substrate for conversion to fibrin, and a scaffold for fibrinolysis and wound healing.1 In keeping with the complexity of the fibrinogen molecule, quantitative and/or qualitative fibrinogen deficiency may cause a wide range of symptoms.2 Several types and subtypes of congenital dysfibrinogenemia (CD) have been identified. For the present purposes, CD is classified according to the International Society of Thrombosis and Hemostasis recommendations (Table 1).3 It is characterized by structural changes in fibrinogen protein that lead to defective fibrin polymerization and/or impaired fibrinolysis.4 CD results from monoallelic mutations in one of the fibrinogen genes (FGA, FGB, or FGG) inherited in autosomal dominant pattern.5 The prevalence of CD is probably underestimated. Indeed, information extracted from the Genome Aggregation Database has shown that the prevalence of CD could be 0.3% in Finns and even 1% in African Americans.6 Since the first patient reported in 19587 and the first mutation identified in 1968,8 hundreds of CD-affected families with various clinical phenotypes have been reported, and more than 100 causative mutations have been studied.9 These observations have given us important insights into our knowledge of fibrinogen structure, the fibrin network, and the mechanisms of fibrin polymerization and fibrinolysis.10,11 They have highlighted the heterogeneity of the clinical features and the difficulty in treating CD, for which both diagnostic and therapeutic strategies remain uncertain. Indeed, we must acknowledge that the management of CD lacks evidence.
International Society of Thrombosis and Hemostasis classification of congenital fibrinogen disorders
| Type and subtypes . |
|---|
| Qualitative |
| 1. Afibrinogenemia |
| A. Patients with a bleeding phenotype or asymptomatic individuals |
| B. Patients with a thrombotic phenotype |
| 2. Hypofibrinogenemia |
| A. Severe: fibrinogen activity level <0.5 g/L |
| B. Moderate: fibrinogen activity level between 0.5 and 0.9 g/L |
| C. Mild: fibrinogen activity level between 1 g/L and the lower limit of normal range of reference |
| D. Fibrinogen storage disease: familial hypofibrinogenemia with histologically proven accumulation of fibrin in hepatocytes |
| Quantitative |
| 3. Dysfibrinogenemia |
| A. Patients with bleeding phenotype or a thrombosis phenotype that does not fulfill the criteria for 3B, or asymptomatic individuals |
| B. Thrombosis-related dysfibrinogenemia: carriers of an established thrombotic fibrinogen mutation (fibrinogen Dusart, fibrinogen Caracas V, fibrinogen Ijmuiden, Fibrinogen New York I, fibrinogen Nijmegen, fibrinogen Naples in the homozygous state, and fibrinogen Melun) or experiencing thrombosis events with a first-degree familial history of thrombosis (relatives with the same genotype) without any other thrombophilia |
| 4. Hypodysfibrinogenemia |
| A. Severe: fibrinogen antigen level <0.5 g/L |
| B. Moderate: fibrinogen antigen between 0.5 and 0.9 g/L |
| C. Mild: fibrinogen antigen level between 1 g/L and the lower limit of normal range of reference |
| Type and subtypes . |
|---|
| Qualitative |
| 1. Afibrinogenemia |
| A. Patients with a bleeding phenotype or asymptomatic individuals |
| B. Patients with a thrombotic phenotype |
| 2. Hypofibrinogenemia |
| A. Severe: fibrinogen activity level <0.5 g/L |
| B. Moderate: fibrinogen activity level between 0.5 and 0.9 g/L |
| C. Mild: fibrinogen activity level between 1 g/L and the lower limit of normal range of reference |
| D. Fibrinogen storage disease: familial hypofibrinogenemia with histologically proven accumulation of fibrin in hepatocytes |
| Quantitative |
| 3. Dysfibrinogenemia |
| A. Patients with bleeding phenotype or a thrombosis phenotype that does not fulfill the criteria for 3B, or asymptomatic individuals |
| B. Thrombosis-related dysfibrinogenemia: carriers of an established thrombotic fibrinogen mutation (fibrinogen Dusart, fibrinogen Caracas V, fibrinogen Ijmuiden, Fibrinogen New York I, fibrinogen Nijmegen, fibrinogen Naples in the homozygous state, and fibrinogen Melun) or experiencing thrombosis events with a first-degree familial history of thrombosis (relatives with the same genotype) without any other thrombophilia |
| 4. Hypodysfibrinogenemia |
| A. Severe: fibrinogen antigen level <0.5 g/L |
| B. Moderate: fibrinogen antigen between 0.5 and 0.9 g/L |
| C. Mild: fibrinogen antigen level between 1 g/L and the lower limit of normal range of reference |
Adapted from Casini et al with permission.3
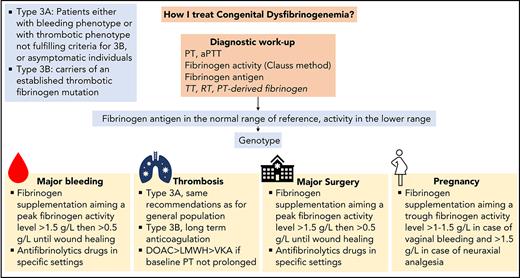
In the light of 5 clinical cases, we discuss the various steps required for the diagnosis of CD and then describe how we currently manage bleeding, thrombosis, surgery, and pregnancy.
Diagnosis of congenital dysfibrinogenemia
Case 1
A low level of fibrinogen activity (0.7 g/L by the Clauss method) was discovered in a healthy 47-year-old woman during general health screening. The initial laboratory findings revealed slightly prolonged prothrombin time (PT) and activated partial thromboplastin time (aPTT) (Table 2). The patient was asymptomatic without previous thrombosis events or bleeding episodes. She was healthy and did not report any medication. She had undergone an appendectomy and tooth extraction in childhood without complications. She had had 2 uneventful pregnancies with vaginal delivery. Blood cell counts and other coagulation factors were all in the normal range. A fibrinogen disorder was suspected, and investigations were completed with measurement of fibrinogen antigen, thrombin time (TT), reptilase time (RT), and genotype, leading to a diagnosis of CD type 3A (Table 2). The patient wondered about the necessity of screening her children.
Fibrinogen workup performed in the investigation of a 47-year-old woman with suspected fibrinogen disorder: case 1
| First-step laboratory diagnosis . | Results . | Reference range . |
|---|---|---|
| PT, s | 13.5 | 9.5-12.7 |
| aPTT, s | 33 | 22-30 |
| Fibrinogen activity, g/L | 0.7 | 1.8-4.0 |
| Second-step laboratory diagnosis | ||
| Fibrinogen antigen, g/L | 3.4 | 1.8-4.0 |
| Thrombin time, s | 46 | 16-21 |
| Reptilase time, s | >60 | 16.21 |
| FGA, FGB, and FGG genotype | FGA c.103C>T p.Arg35His* | Causative hotspot mutation |
| First-step laboratory diagnosis . | Results . | Reference range . |
|---|---|---|
| PT, s | 13.5 | 9.5-12.7 |
| aPTT, s | 33 | 22-30 |
| Fibrinogen activity, g/L | 0.7 | 1.8-4.0 |
| Second-step laboratory diagnosis | ||
| Fibrinogen antigen, g/L | 3.4 | 1.8-4.0 |
| Thrombin time, s | 46 | 16-21 |
| Reptilase time, s | >60 | 16.21 |
| FGA, FGB, and FGG genotype | FGA c.103C>T p.Arg35His* | Causative hotspot mutation |
Nascent protein with the signaling peptide.
Discussion of case 1
Incidental finding is the most common cause of diagnosis in about half of cases of CD.12-17 Nevertheless, asymptomatic patients at the time of diagnosis may develop symptoms during their clinical course. Therefore, all patients with suspected CD should be referred to a hemophilia treatment center to secure the diagnosis, perform a family screening, and determine a comprehensive treatment plan in case of an emergency or an elective surgery.
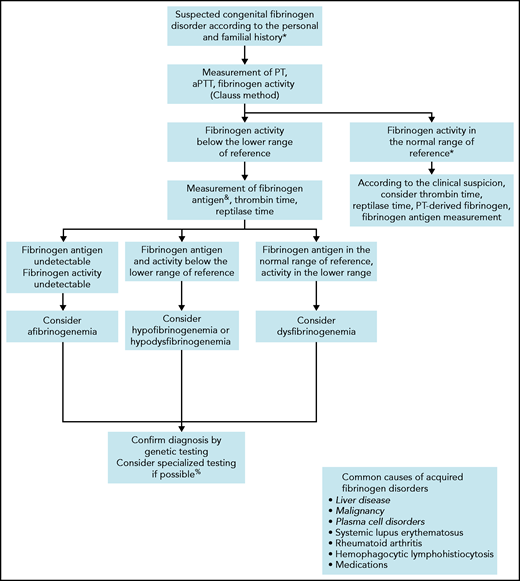
Before considering a congenital fibrinogen disorder in an asymptomatic patient, nonhereditary causes should be ruled out (Figure 1). Liver disease is the most common cause of acquired CD, leading to an increased sialylation of fibrinogen Bβ and γ chains and resulting in a delay of fibrin polymerization.18,19 In addition, paraproteinemia,20,21 paraneoplastic synthesis of abnormal fibrinogen,22 or autoantibodies23 may interfere with fibrinogen function. Distinguishing acquired dysfibrinogenemia from CD is usually simple according to the clinical context, family history, and association with other laboratory abnormalities.
Algorithm for the diagnosis of a suspected congenital fibrinogen disorder.
Algorithm for the diagnosis of a suspected congenital fibrinogen disorder.
When a fibrinogen disorder is suspected, we follow the steps indicated in Figure 1 to characterize the type of disorder. If they have not already been performed, we start with assessments of PT, aPTT, and fibrinogen clotting ability (ie, activity). Fibrinogen activity should be measured initially by the functional Clauss assay, which consists of the measurement of the clotting time of plasma after activation with a high concentration of thrombin.24 The sensitivity of the Clauss method (as well of PT and aPTT) as a screen for CD varies according to the reagents,25 the end-point detection systems (ie, mechanical or photo-optical),14 and the causative fibrinogen mutation.26 Another possibility is the PT-derived method, which is an indirect measurement of fibrinogen activity based on the change in light scatter or optical density during measurement of PT.27 However, this alternative is not suitable for the screening of CD, because it overestimates fibrinogen activity by approximately sixfold compared with the Clauss assay.28 Nevertheless, rare fibrinogen variants such as fibrinogen Longmont (Bβ p.Arg166Cys, protein with peptide signal),29,30 or fibrinogen Bordeaux (Aα p.Arg439Cys),31 can be missed by the Clauss assay but revealed by the PT-derived method.26 Thus, in unexplained cases of bleeding with a strong suspicion of fibrinogen disorder, we consider the PT-derived fibrinogen as an additional screening method.
As a second diagnostic step, we estimate fibrinogen antigen levels to determine the type of fibrinogen disorder. The clottable protein method was the reference assay for estimation of fibrinogen concentration, but it is now hardly ever used because it is technically difficult and time-consuming. Assays based on enzyme-linked immunoabsorbent assay or radial immunodiffusion are the ones most commonly used today.27 Discordance between decreased levels of fibrinogen activity and normal levels of fibrinogen antigen is highly suggestive of CD.32 A ratio of fibrinogen activity to antigen <0.7 is generally accepted as diagnostic of CD,33 even though this cutoff has not been validated.34 If an immunoassay is not available, we propose the PT-derived method as an alternative in view of its good correlation with fibrinogen antigen (r = 0.86-0.93).28,35 A ratio of PT-derived fibrinogen to Clauss >1.7 is highly sensitive and specific for CD.36 Although it is not mandatory for the diagnosis of CD, we usually complete the second step with measurements of TT and RT. These coagulation times are variably prolonged, but can provide additional information on the polymerization defects.37
Finally, we perform genetic testing to confirm the diagnosis and facilitate family screening. Genetic counseling is eventually provided to affected relatives. Up to 85% of causative mutations identified in CD are heterozygous missense mutations clustered in exon 2 of FGA or exon 8 of FGG.38 Among them, the 2 most frequently mutated residues (ie, hotspot mutations) are Aα p.Arg35Cys/His and γ p.Arg301Cys/His.9 Although there is no specific correlation between the genotype and the clinical phenotype for most fibrinogen variants, some are strongly predictive of thrombotic disease (discussed in case 3).39,40 In addition, specialized research laboratories may individualize the fibrinogen workup by analyzing the fibrin network and fibrin clot properties, to better assess the patient’s phenotype.41-44
Management of acute bleeding in congenital dysfibrinogenemia
Case 2
A 37-year-old woman known for type 3A CD (hotspot mutation, Aα p.Arg35Cys; baseline fibrinogen activity, 0.6 g/L; fibrinogen antigen, 2.6 g/L; TT and RT, >60 seconds) reported a sudden pain in the right thigh after a trauma. She had difficulty standing and walking and observed a large ecchymosis along the medial side of her thigh. Because the clinical symptoms worsened, she went to the emergency room. Her physical examination was remarkable only for tachycardia and a large hematoma in the thigh region. Laboratory results were in the normal range, except for decreased hemoglobin (107 g/L), a prolonged PT (17.5 s), and low fibrinogen activity (0.4 g/L by the Clauss method). Ultrasound confirmed a tear of the gracilis muscle with a large hematoma. An acute compartment syndrome was ruled out. Rest, ice, and compression were immediately started, and 3 g fibrinogen concentrate (50 mg/kg) was administered. The peak fibrinogen activity level was measured at 1.6 g/L 1 hour after the infusion. The day after, fibrinogen activity, fibrinogen antigen, TT, and RT were 1.3 g/L, 3.1 g/L, 32 seconds, and 36 seconds, respectively. Further infusions of fibrinogen concentrate (2 g) were administered on day 4 (trough fibrinogen activity 0.7 g/L) and on day 8 at discharge.
Discussion of case 2
To different degrees, all cases of CD have defective fibrin polymerization and thereby abnormal clottability.45 CD is usually considered to be a mild bleeding disorder, because mild bleeding episodes are the ones most often reported by patients.46 Bleeding may be spontaneous (mainly cutaneous and heavy menstrual bleeding), but is usually provoked by significant hemostatic challenges, such as surgery or trauma,12,16,47 corresponding to grade 1 or 2 bleeding according to classification in the European Network of Rare Bleeding Disorders.48 In a large cohort of patients (n = 101), at least 1 bleeding symptom was reported in 48 (47.5%), with a mean International Society on Thrombosis and Haemostasis bleeding assessment tool score of 1.6.13 Major bleeding, especially postpartum hemorrhage (PPH), is less frequent but still occurs, with an incidence estimated at 3.5 per 1000 patient years.13
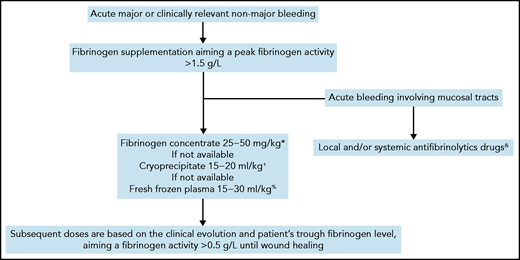
Currently, there are no evidence-based recommendations for the management of CD.49 Depending on their availability, fresh frozen plasma, cryoprecipitate, or plasma-derived fibrinogen concentrate can be administered to increase fibrinogen levels.50 The last treatment is preferred in view of its more favorable safety and efficacy profiles.51 The optimal threshold of fibrinogen activity in CD level in cases of acute bleeding is unknown and is mainly extrapolated from that established for bleeding in other types of fibrinogen disorders.5,52 Although it is debatable, a threshold fibrinogen activity level of 1 to 1.5 g/L has historically been used for fibrinogen supplementation in patients with quantitative or acquired fibrinogen deficiencies.49,53-55 The clot strength increases linearly with fibrinogen concentration, with a minimum level of 2.0 g/L required in vitro for the optimal rate of clot formation to be achieved.56 To facilitate and harmonize management, we usually raise peak fibrinogen levels to >1.5 g/L for most major or clinically relevant nonmajor bleeding events (Figure 2). Subsequent doses are based on the clinical evolution and the patient’s trough fibrinogen activity level, aiming at ≥0.5 g/L throughout wound healing.49 The interindividual pharmacokinetics of fibrinogen concentrate is variable,57 but the long half-life of fibrinogen (median, 3-5 days) usually enables infusion twice a week or less outside an acute setting.58 Importantly, when fibrinogen concentrates are administered to a patient with dysfibrinogenemia, a mixture of fully functional fibrinogen and dysfunctional fibrinogen will circulate. The functional properties of fibrin clots composed of these two types of fibrinogen are unknown.19,59 When monitoring fibrinogen supplementation in CD, we measure the fibrinogen antigen concentration to evaluate the overall quantity of circulating fibrinogen and also the TT and RT, to assess the improvement of the polymerization defect. Recently, it has been proposed that a correction factor derived from TT-mixing studies can be used to modify the approaches to fibrinogen supplementation in CD.37 However, this interesting method must be validated in large cohorts before it can be considered for guiding fibrinogen supplementation. In cases with bleeding involving mucous membranes, we add a local or systemic antifibrinolytic agent for up to 10 days, provided that the patient does not have thrombosis-related dysfibrinogenemia (type 3B, see case 3).
Algorithm for management of acute bleeding in congenital dysfibrinogenemia.
Algorithm for management of acute bleeding in congenital dysfibrinogenemia.
Management of thrombosis in congenital dysfibrinogenemia
Case 3
A 33-year-old-man was referred to the emergency room due to swelling, tenderness, and erythema in the left leg for 3 days. He was known to have type 3A CD (hotspot mutation, γ p.Arg301Cys) diagnosed after a family screening. Deep venous thrombosis was suspected on the basis of elevated D-dimers (1600 ng/mL) and was confirmed by positive ultrasonography (thrombosis in femoral and popliteal veins). He did not report any history of immobilization, hospitalization, cancer, trauma, or recent surgery. The family history was remarkable only for a mild bleeding phenotype in his father and brother, both carriers of the fibrinogen mutation. The patient reported no bleeding episodes so far, and he denied any hemostatic challenge. Laboratory testing revealed a normal complete blood count, decreased fibrinogen activity (0.9 g/L by the Clauss method) with a normal antigen level (3.4 g/L), a slightly prolonged PT and aPTT, normal inherited thrombophilia screening results, and an absence of antiphospholipid antibodies. Treatment included outpatient anticoagulation with a direct oral anticoagulant.
Discussion of case 3
Besides the bleeding risk, CD may increase the thrombosis risk illustrating the versatile function of fibrinogen in coagulation. The annual incidence of arterial and venous thrombosis in CD has been estimated to be 13.9 per 1000 adult patient-years without a difference between the sexes.13 In addition, some thrombosis-related variants (types 3B) have clearly been identified to confer a higher risk of thrombosis (Table 1). Overall, CD type 3A may be associated with a thrombosis risk, whereas type 3B should be regarded as severe thrombophilia. Different mechanisms, mostly overlapping, contribute to the general thrombosis risk in CD, such as defective binding of thrombin,60 plasminogen,61 or tissue-plasminogen activator62 by the fibrinogen variant; impaired viscoelastic parameters; and altered fibrin network.63 Both venous and arterial territories can be involved, as well as unusual vascular sites.64,65 The penetrance of the thrombosis phenotype is often incomplete, suggesting that additional underlying mechanisms contribute to the overall thrombosis risk.66 The value of D-dimer in the diagnosis algorithm of venous thromboembolism (VTE) in CD has not been specifically assessed.
For the treatment of provoked or unprovoked VTE, we generally adopt the same recommendations as for the general population (Table 3).67 Even though data on efficacy and security of direct oral anticoagulation in that setting are still scarce,16,65,68 we often start with one of these anticoagulants as a first-line option. As the second choice, we use low-molecular-weight heparin rather than vitamin K antagonists, because the international normalized ratio may not be a valid measurement in cases with prolonged baseline PT.4 The decision regarding the optimal duration of anticoagulation should involve an accurate evaluation of the additional persistent thrombosis risk factors and individual preference. Because of the risk of bleeding, we usually favor a limited duration of anticoagulation (ie, 3-6 months) in 3A CD rather than a longer one. For type 3B CD, we propose long-term anticoagulation.
General principles and modalities of management of thrombosis in congenital dysfibrinogenemia: case 3
| Investigation of thrombosis • Screening of CD should be considered as a second- or third-line investigation in selected patients and families after having ruled out the more common causes of thrombophilia. |
| Treatment of venous thrombosis • For patients with type 3A dysfibrinogenemia, we adopt the same recommendations as for the general population, favoring a limited duration of anticoagulation (ie, 3-6 mo). • For patients with type 3B dysfibrinogenemia, we propose a long-term anticoagulation. • Anticoagulation with a direct anticoagulant is our first choice. Low-molecular-weight heparin is the second choice. A vitamin K antagonist may be considered if the baseline PT is not prolonged. |
| Thromboprophylaxis • For patients with type 3A dysfibrinogenemia we adopt the same recommendations as for the general population, favoring a mechanical thromboprophylaxis whenever possible. • For patients with type 3B dysfibrinogenemia, we adopt the same recommendations as for the general population favoring a pharmacological thromboprophylaxis, whenever possible. |
| Investigation of thrombosis • Screening of CD should be considered as a second- or third-line investigation in selected patients and families after having ruled out the more common causes of thrombophilia. |
| Treatment of venous thrombosis • For patients with type 3A dysfibrinogenemia, we adopt the same recommendations as for the general population, favoring a limited duration of anticoagulation (ie, 3-6 mo). • For patients with type 3B dysfibrinogenemia, we propose a long-term anticoagulation. • Anticoagulation with a direct anticoagulant is our first choice. Low-molecular-weight heparin is the second choice. A vitamin K antagonist may be considered if the baseline PT is not prolonged. |
| Thromboprophylaxis • For patients with type 3A dysfibrinogenemia we adopt the same recommendations as for the general population, favoring a mechanical thromboprophylaxis whenever possible. • For patients with type 3B dysfibrinogenemia, we adopt the same recommendations as for the general population favoring a pharmacological thromboprophylaxis, whenever possible. |
It should be noted that the prevalence of CD in patients with thrombosis events is low.69 Therefore, we do not recommend systematic testing for CD in cases of VTE, except as second- or third-line testing in patients with a strong personal and/or family history of thrombosis after the more common causes of thrombophilia have been ruled out.70
Management of elective surgery in congenital dysfibrinogenemia
Case 4
An 18-year-old man was referred by his general practitioner for a wisdom tooth extraction. A diagnosis of CD (type 3A) was made after investigation of his tendency toward bruising in exposed areas and epistaxis requiring recurrent cauterizations. He had undergone no previous surgery. Recent analyses confirmed low fibrinogen activity (0.6 g/L by the Clauss method) and normal fibrinogen antigen (2.6 g/L). The patient’s mother and sister had a similar biological phenotype. His mother experienced a major bleeding after a tooth extraction and a PPH, whereas his sister was asymptomatic. In view of his bleeding phenotype and the family history, infusion of 2 g of fibrinogen concentrate (40 mg/kg) was proposed 1 hour before surgery, aiming for fibrinogen activity of 1.5 g/L, followed by 1 g tranexamic acid 3 times per day for 10 days.
Discussion case 4
Among 137 surgeries in the aforementioned large cohort of patients with CD, 9 (6.5%) were complicated by abnormal bleeding including 4 major episodes.13 Apart from a few case reports, fibrinogen supplementation in preventing surgery-related bleeding has essentially been evaluated in quantitative fibrinogen disorders.71 Overall, convincing data from clinical trials in afibrinogenemia support the efficacy and safety of fibrinogen concentrates in this setting.72-74 In the postsurgical period, fibrinogen plays a major role in wound healing by promoting tissue regeneration.75
As a general rule, we establish the preoperative planning with surgeons and anesthetists, taking into account the patient’s personal and family history. Preoperative assessment includes evaluation of baseline fibrinogen activity and antigen. Surgery is usually scheduled according to the availability of laboratory and blood bank support. Adequate quantities of fibrinogen should be available for the surgery and throughout wound healing. Depending on the surgeon’s estimation of the extent and complexity of the procedure and the patient’s comorbidities, we usually consider most orthopedic, abdominal, gynecological, urological, neurological, thoracic, and otolaryngology surgeries to be major interventions requiring preventive fibrinogen supplementation. In such cases, we administer fibrinogen, targeting a perioperative peak activity >1.5 g/L. An approach based on viscoelastic parameters from rotational thromboelastometry or thromboelastography has not been studied in the perioperative setting of CD.76 Subsequent doses are based on the clinical evolution and the patient’s trough fibrinogen levels, aiming at a fibrinogen activity level >0.5 g/L until the wound heals (Figure 3).49 For minor surgeries, including dental extractions, we generally avoid preventive fibrinogen supplementation, but use it in cases of unusual perioperative bleeding. Of note, we administer preventive fibrinogen in patients with a bleeding phenotype, even for minor surgery (as in case 3) or in asymptomatic patients without previous hemostatic challenges. Antifibrinolytics are added for mucosal or dental procedures in patients without thrombosis-related dysfibrinogenemia. Thromboprophylaxis (mechanical or pharmacological) should be tailored according to the specific surgical procedure, thrombosis risk, and bleeding tendency. We recommend avoiding intake of nonsteroidal anti-inflammatory drugs in the perioperative period.
Algorithm for management of elective surgery in congenital dysfibrinogenemia.
Algorithm for management of elective surgery in congenital dysfibrinogenemia.
Management of dysfibrinogenemia during pregnancy
Case 5
A 27-year-old woman, diagnosed with hereditary dysfibrinogenemia (γ p.Arg301His) after investigation of heavy menstrual bleeding since menarche, was referred for vaginal bleeding. She was at 16 weeks of gestation in her first pregnancy. At admission, fibrinogen activity was measured at 0.6 g/L (by the Clauss method) and fibrinogen antigen at 3.3 g/L. Sonographic findings were consistent with a retroplacental hematoma. Fibrinogen supplementation with fibrinogen concentrate was begun, to achieve a trough fibrinogen level >1.5 g/L. Vaginal bleeding ended at 19 weeks of gestation. At 35 weeks of gestation, fibrinogen activity and antigen levels were 1.4 and 4.4 g/L, respectively. A cesarean delivery was planned due to breech presentation. Neuraxial anesthesia was used after fibrinogen supplementation, leading to a fibrinogen activity level of 1.7 g/L. The delivery was unremarkable with immediate postpartum fibrinogen activity at 1.2 g/L. A mechanical postpartum thromboprophylaxis was administrated according to standard recommendations.
Discussion of case 5
Pregnancy is a high-risk clinical situation in women who have CD.77 Miscarriage,78 stillbirth,13 placenta abruptio,79 intrauterine growth restriction,80 PPH,81 and thrombosis82 have been reported, although most pregnancies progress smoothly. In a cohort of 111 pregnancies, the miscarriage rate was 19.8%,13 even though, in the subgroup of women with a thrombosis-related mutation, the rate rose significantly to about 40%.39 The risk of PPH also seems to be increased. In the above-mentioned cohort of 111, 21.4% of the pregnancies were complicated by PPH. In a multivariate analysis adjusted for age and parity, a previous bleeding phenotype was associated with an increased risk of PPH (odds ratio, 5.8; 95% confidence interval, 0.2-0.3; P < .03).13 Of note, fibrinogen activity and antigen levels physiologically increase during pregnancy but no data are available on fibrinogen level variations in the setting of CD.
Pregnancy should be managed by a multidisciplinary team consisting of obstetricians, hematologists, and anesthetists (Table 4).83 Preconception counseling should ideally provide adequate information on the clinical and genetic implications of CD and the management of the pregnancy. Throughout the pregnancy, we perform a systematic ultrasound assessment to monitor fetal growth and a quarterly measurement of fibrinogen activity (and fibrinogen antigen, if measurement is available) to monitor the increase in fibrinogen. We do not administer fibrinogen to asymptomatic women who have no history of obstetrical complications. As in this case, we reserved fibrinogen supplementation for treatment of vaginal bleeding, targeting a fibrinogen activity >1.5 g/L until resolution of the bleeding. We also discussed fibrinogen supplementation in cases of intrauterine growth restriction caused by placental dysfunction without identified cause. For recurrent miscarriages, fibrinogen supplementation throughout the pregnancy can be considered. A point of debate is the level of fibrinogen activity to target. Some researchers have suggested an activity level >1 g/L.78,79 However, given the physiological increase in fibrinogen throughout the pregnancy (and observations from afibrinogenemia), a more intensive supplementation may be needed, aiming for fibrinogen activity >1.5 g/L, at least in the third trimester.84 In women with a history of thrombosis or a type 3B CD, we introduce pharmacological thromboprophylaxis early in pregnancy without preventive fibrinogen supplementation. The optimal mode of delivery is not driven by the fibrinogen level but rather by fetal and maternal risks. However, we suggest a cesarean delivery when an operative vaginal delivery fails, to avoid invasive fetal procedures and forceps- or vacuum-assisted delivery. According to the patient’s choice, we encourage neuraxial anesthesia for labor analgesia after fibrinogen supplementation, targeting fibrinogen activity >1.5 g/L. The risk of PPH is of major concern. Thus, maternal fibrinogen activity levels should be monitored after birth, and early fibrinogen supplementation and antifibrinolytic agents should be prescribed in case of bleeding. We manage postpartum thromboprophylaxis according to the same recommendations as those for the general population, favoring mechanical thromboprophylaxis in women with type 3A CD and pharmacological thromboprophylaxis in women with type 3B CD, usually during the 6 weeks after birth.85,86
General principle and modalities of management of pregnancy in congenital dysfibrinogenemia: case 5
| Preconception counseling • Provide adequate information on potential clinical complications and implications of genetic screening. • Discuss pregnancy management. |
| Antenatal management • Quarterly assessment of fibrinogen activity level. • Systematic monitoring of fetal growth. • Fibrinogen supplementation targeting a fibrinogen activity trough level >1 to 1.5 g/L in cases with vaginal bleeding. |
| Labor and delivery • Fibrinogen supplementation targeting a fibrinogen activity peak level >1.5 g/L in cases with neuraxial analgesia. • Avoid invasive fetal procedures and forceps- or vacuum-assisted delivery. |
| Postpartum management • Monitoring of fibrinogen activity level. • Early fibrinogen supplementation and an antifibrinolytic drug in case of postpartum hemorrhage. • Thromboprophylaxis according to the same recommendation as for the general population, favoring a mechanical thromboprophylaxis whenever possible in patients with type 3A CD. • For patients with type 3B dysfibrinogenemia, adopt the same recommendations as for the general population, favoring pharmacological thromboprophylaxis whenever possible. |
| Preconception counseling • Provide adequate information on potential clinical complications and implications of genetic screening. • Discuss pregnancy management. |
| Antenatal management • Quarterly assessment of fibrinogen activity level. • Systematic monitoring of fetal growth. • Fibrinogen supplementation targeting a fibrinogen activity trough level >1 to 1.5 g/L in cases with vaginal bleeding. |
| Labor and delivery • Fibrinogen supplementation targeting a fibrinogen activity peak level >1.5 g/L in cases with neuraxial analgesia. • Avoid invasive fetal procedures and forceps- or vacuum-assisted delivery. |
| Postpartum management • Monitoring of fibrinogen activity level. • Early fibrinogen supplementation and an antifibrinolytic drug in case of postpartum hemorrhage. • Thromboprophylaxis according to the same recommendation as for the general population, favoring a mechanical thromboprophylaxis whenever possible in patients with type 3A CD. • For patients with type 3B dysfibrinogenemia, adopt the same recommendations as for the general population, favoring pharmacological thromboprophylaxis whenever possible. |
Conclusions
The diagnosis and treatment of patients with CD continues to pose significant challenges. The diagnosis should be based on the measurement of fibrinogen activity and antigen with a confirmation by genotyping. The clinical management should be based primarily on the patient’s personal and family bleeding history and/or thrombosis complications. Indeed, most patients are at risk of bleeding or thrombosis complications during their lives. The mainstay of CD treatment remains fibrinogen supplementation. Antifibrinolytic agents should be considered according to the type of bleeding. In the presence of thrombosis, in most CD cases, the same recommendations apply as for the general population. Surgery and plans for pregnancy should be made in conjunction with a multidisciplinary team and an expert coagulation laboratory. Additional adequately powered studies are needed to optimize the guidelines for treating patients with CD.
Acknowledgments
The authors thank Françoise Bridey, Philippe Halban, and Pierre Fontana for lending their expertise and for critical review of the manuscript.
Authorship
Contribution: A.C. and P.d.M. designed the concept and wrote and reviewed the manuscript.
Conflict-of-interest disclosure: A.C. reports grants and fees paid to his institution from CSL Behring, Octapharma, Sobi, Shire, Takeda, and Novo Nordisk A/S. P.d.M. has received grants and fees from Bayer, CSL Behring, LFB, Novo Nordisk A/S, Octapharma, and Shire and has served on advisory boards for Bayer and LFB.
Correspondence: Alessandro Casini, Division of Angiology and Hemostasis, University Hospitals of Geneva, Rue Gabrielle-Perret-Gentil 4, 1205 Geneva, Switzerland; e-mail: alessandro.casini@hcuge.ch; and Philippe de Moerloose, Faculty of Medicine, Rue Michel Servet 1, 1205 Geneva, Switzerland; e-mail: philippe.demoerloose@unige.ch.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal