Key Points
Fixed-duration combination treatment including venetoclax or ibrutinib plus obinutuzumab can induce deep remissions in high-risk CLL.
Responses are also durable after MRD-guided discontinuation of treatment.
Abstract
Fifty-one of 189 evaluable patients from 3 prospective phase 2 trials evaluating a sequential targeted treatment had high-risk chronic lymphocytic leukemia (CLL) with a 17p deletion, TP53 mutation, or both. Twenty-seven patients started treatment with bendamustine debulking before induction and maintenance treatment, which was ibrutinib/ofatumumab (IO) in 21 patients, ibrutinib/obinutuzumab (IG) in 13, and venetoclax/obinutuzumab (AG) in 17. The primary end point was overall response rate after 8 months of induction treatment, which was 81%, 100%, and 94% for IO, IG, and AG, respectively. Minimal residual disease (MRD) was undetectable (uMRD) in peripheral blood (<10−4 by flow cytometry) in 0%, 23%, and 82% of patients, respectively. Median progression-free survival (PFS) was 45 months. Seventeen patients discontinued maintenance treatment due to uMRD: 9 progressed, 2 died without progression (median PFS, 28 months after discontinuation of treatment), and 6 remained in remission after a median observation time of 46 months (range, 6-47 months) after treatment discontinuation. Thus, MRD-guided fixed-duration therapies combining obinutuzumab with venetoclax or ibrutinib can induce deep and durable remissions in CLL patients with high-risk genetic lesions, which can persist after treatment discontinuation (due to a predefined fixed-duration or MRD-guided early termination). The median PFS was 45 months. These trials were registered at www.clinicaltrials.gov as #NCT02345863, #NCT02401503, and #NCT02689141.
Introduction
The introduction of targeted agents has revolutionized the treatment of patients with chronic lymphocytic leukemia (CLL) and especially of patients harboring a 17p deletion [del(17p)] or TP53 mutation (TP53 mut).1,2 The Bruton tyrosine kinase (BTK) inhibitor ibrutinib has become the standard treatment of patients with high-risk disease and is also increasingly used for other patient groups.3-9 However, complete remissions (CRs) and minimal residual disease (MRD) with undetectable (uMRD) levels occur infrequently, and continuous treatment appears to be necessary for disease control.4,6,10-14 Prolonged treatment duration increases the risk of toxicities and patients’ noncompliance, as well as the selection of resistant CLL clones, and the costs of treatment. The B-cell lymphoma 2 (BCL-2) inhibitor venetoclax achieves deeper remissions, especially in combination with an anti-CD20 antibody, and was established with a fixed treatment duration.15-20
The so-called “sequential triple-T concept” of a tailored and targeted treatment aiming at a total eradication of MRD introduced the idea of a sequential treatment regimen combining different agents to achieve MRD “negative” CRs with an eradication of disease below the detection limit, which could potentially enable treatment discontinuations.21,22
Based on the sequential triple-T concept, a series of phase 2 trials was designed each evaluating an optional debulking with bendamustine followed by a targeted agent plus an anti-CD20 antibody in an all-comer collective irrespective of prior treatment, physical fitness, and genetic risk factors. Three of these trials have reached their primary end point: ibrutinib (I) was combined with ofatumumab (O [IO]) in the CLL2-BIO trial; ibrutinib (I) was combined with obinutuzumab (G [IG]; for GA101) in the CLL2-BIG trial; and venetoclax (A; for ABT-199) was combined with obinutuzumab (G [AG]) in the CLL2-BAG trial. The overall response rate (ORR) at the end of induction treatment was 92% for IO, 100% for IG, and 95% for AG. The corresponding rates of uMRD by flow cytometry in peripheral blood were 14%, 48%, and 87%, respectively.23-25 Patients achieving a deep response with uMRD levels discontinued treatment as intended by the trial protocols.
To assess whether treatment discontinuations are feasible without an immediate relapse, all patients with a del(17p) and/or TP53 mut from the 3 trials were pooled for this analysis to assess whether a fixed-duration treatment is feasible in a population with the highest risk of an early relapse.
Methods
The 3 prospective, multicenter phase 2 studies were run by the German CLL Study Group (GCLLSG) as investigator-initiated trials with the University of Cologne (Cologne, Germany) being the legal sponsor. The studies were approved by the health authorities and the institutional review board of each participating site, and all patients provided written informed consent. Between January 2015 and October 2016, a total of 189 evaluable patients were enrolled in the 3 trials: 65 patients received IO in the CLL2-BIO trial, 61 patients received IG in the CLL2-BIG trial, and 63 patients received AG in the CLL2-BAG trial.
The trials were designed for all-comer populations of physically fit and unfit patients with CLL requiring treatment according to International Workshop on Chronic Lymphocytic Leukemia (iwCLL) criteria,26 irrespective of number and type of prior therapies, as well as genetic risk profile and burden of comorbidities. In addition to standard eligibility criteria, such as adequate renal, hepatic, and hematological function and exclusion of active infections, certain comorbidities and concomitant medications had to be excluded to avoid excessive toxicity, for example, use of strong CYP3A4/5 inhibitors/inducers and vitamin K antagonists. Further details regarding the trial designs were previously published.23-25
In all 3 trials, a debulking with bendamustine (70 mg/m2 on days 1 and 2 of two 28-day cycles) was recommended for patients with a relevant tumor burden (absolute lymphocyte count >25 000/µL and/or lymph nodes >5 cm) if no contraindications were present. In accordance with the established administration schedules of the individual drugs, the anti-CD20 antibodies were administered 3 times as a single agent in the first cycle (obinutuzumab 1000 mg on days 1, 2, 8, and 15; ofatumumab 300 mg on day 1 and 1000 mg on days 8 and 15 of cycle 1) and monthly thereafter (both 1000 mg day 1 of cycles 2-6). The continuous intake of the oral targeted agent started in cycle 2 (ibrutinib 420 mg daily; venetoclax with a weekly dose ramp-up in 5 steps from 20 mg to 400 mg and with the established safety precautions for prevention/early detection of a tumor lysis syndrome). In the maintenance phase, the oral agent was continued daily, but the administration intervals of the antibody were extended to every three months. Maintenance treatment was continued for up to 24 months or until achievement of a deep response with a uMRD level, until inacceptable toxicity, progression of CLL, subsequent treatment, or withdrawal of consent (see also supplemental Figure 1, available on the Blood Web site).
At baseline, diagnosis of CLL was confirmed centrally by immunophenotyping and all established risk factors were assessed centrally, including fluorescence in situ hybridization (FISH) cytogenetics (for del(17p) a test-specific cutoff of 7.4% was used) and molecular genetics with TP53 mut (cutoff was set to 5% variant allele frequency) in the laboratory at the University in Ulm. Blood samples were also collected at the time of progression for repetition of FISH and next-generation sequencing analyses on recurrent mutated genes including TP53, BTK, PLCG2, and BCL2. MRD evaluations by 4-color flow cytometry were performed from the final restaging onward in the reference laboratory at the University in Kiel.27,28 Results were categorized into 3 different MRD levels: undetectable/low (<10−4), intermediate (≥10−4 and <10−2), and high (≥10−2).29
The primary end point of the trials was the ORR at the end of induction therapy (after 8 months of targeted treatment), which was tested vs the null hypothesis of 75% via the binomial test. Secondary end points included MRD evaluations, safety, and survival parameters.
For this analysis, only patients with a del(17p)/TP53 mut from the 3 trials were pooled; data cutoff was 20 May 2019 for CLL2-BIG, 14 October 2020 for CLL2-BIO (final data sets), and 17 February 2021 for CLL2-BAG (Table 1). Twenty-one patients were followed in the GCLLSG registry; additional follow-up from this source was used with the data cutoff 17 February 2021.
Overview of the 3 trials and patients included in the analysis
| . | CLL2-BIO . | CLL2-BIG . | CLL2-BAG . | Total . |
|---|---|---|---|---|
| Evaluable patients | 65 | 61 | 63 | 189 |
| ORR at end of induction, % | 92 | 100 | 95 | NA |
| uMRD level (<10−4) at end of induction, % | 14 | 48 | 87 | NA |
| Data cutoff | 14 October 2020 | 20 May 2019 | 17 February 2021 | NA |
| Observation time, median (range), mo | 38 (8-43) | 38 (5-49) | 38 (8-56) | 38 (5-56) |
| Patients with del(17p)/TP53 mutation | 21 | 13 | 17 | 51 |
| Observation time, median (range), mo | 45 (8-55) | 41 (21-67) | 61 (8-68) | 49 (8-68) |
| . | CLL2-BIO . | CLL2-BIG . | CLL2-BAG . | Total . |
|---|---|---|---|---|
| Evaluable patients | 65 | 61 | 63 | 189 |
| ORR at end of induction, % | 92 | 100 | 95 | NA |
| uMRD level (<10−4) at end of induction, % | 14 | 48 | 87 | NA |
| Data cutoff | 14 October 2020 | 20 May 2019 | 17 February 2021 | NA |
| Observation time, median (range), mo | 38 (8-43) | 38 (5-49) | 38 (8-56) | 38 (5-56) |
| Patients with del(17p)/TP53 mutation | 21 | 13 | 17 | 51 |
| Observation time, median (range), mo | 45 (8-55) | 41 (21-67) | 61 (8-68) | 49 (8-68) |
NA, not applicable
Results
Fifty-one patients with the genetic high-risk features del(17p) and/or TP53 mut were treated in the 3 trials; 29 patients had both, 20 had a TP53 mut, and 2 a del(17p) only. The majority of the patients also had other risk factors, such as an unmutated immunoglobulin heavy chain variable region (IGHV) status (38 patients; 75%) or complex karyotype (23 of 36 tested patients; 64%). Full baseline characteristics are presented in Table 2.
Baseline characteristics
| . | CLL2-BIO . | CLL2-BIG . | CLL2-BAG . | Total . |
|---|---|---|---|---|
| Analysis population (patients with del(17p)/TP53 mut | 21 | 13 | 17 | 51 |
| Age, y | ||||
| Median (range) | 68 (49-81) | 67 (55-81) | 56 (42-73) | 64 (42-81) |
| Patients aged >65 (%) | 12 (57) | 7 (54) | 3 (18) | 22 (43) |
| Sex | ||||
| Male patients (%) | 14 (67) | 6 (46) | 12 (71) | 32 (63) |
| ECOG performance status 1 or 2 (%) | 12 (57) | 5 (38) | 7 (41) | 24 (47) |
| CIRS comorbidity score | ||||
| Median (range) | 4 (1-9) | 4 (0-10) | 2 (0-14) | 3 (0-14) |
| Patients with CIRS >6 (%) | 4 (19) | 3 (23) | 1 (6) | 8 (16) |
| Creatinine clearance | ||||
| Median, mL/min | 80.5 | 77.8 | 79.9 | 79.9 |
| Patients with creatinine clearance <70 mL/min (%) | 6 (29) | 4 (31) | 5 (29) | 15 (29) |
| Previous therapies | ||||
| Patients with ≥1 prior therapy (%) | 13 (62) | 9 (69) | 11 (65) | 33 (65) |
| Patients with prior treatment, median (range) | 2 (1-5) | 1 (1-5) | 3 (1-9) | 2 (1-9) |
| Binet stage (%) | ||||
| A | 5 (24) | 2 (15) | 7 (41) | 14 (28) |
| B | 8 (38) | 2 (15) | 3 (18) | 13 (26) |
| C | 8 (38) | 9 (69) | 7 (41) | 24 (47) |
| Presence of B symptoms (%) | 9 (43) | 3 (23) | 8 (47) | 20 (39) |
| Serum parameters (%) | ||||
| Thymidine kinase >10 U/L | 19 (95) | 12 (92) | 16 (94) | 47 (94) |
| β2-microglobulin >3.5 mg/L | 13 (62) | 7 (54) | 9 (53) | 29 (58) |
| FISH cytogenetics, Döhner model (%) | ||||
| del(17p) | 12 (57) | 8 (62) | 11 (65) | 31 (61) |
| del(11q) | 2 (10) | 0 | 2 (12) | 4 (8) |
| Trisomy 12 | 0 | 0 | 1 (6) | 1 (2) |
| Normal | 4 (19) | 3 (23) | 2 (12) | 9 (18) |
| del(13q) | 3 (14) | 2 (15) | 1 (6) | 6 (12) |
| Molecular genetics (%) | ||||
| IGHV unmutated | 14 (67) | 9 (69) | 15 (88) | 38 (75) |
| TP53 mutated | 20 (95) | 12 (92) | 17 (100) | 49 (96) |
| NOTCH1 mutated | 2 (10) | 2 (15) | 2 (12) | 6 (12) |
| SF3B1 mutated | 4 (19) | 2 (15) | 3 (18) | 9 (18) |
| Complex karyotype, ≥3 aberrations (%) | 13 (68)* | NK* | 10 (59) | 23 (64)* |
| CLL-IPI (%) | ||||
| High | 3 (15) | 3 (23) | 5 (29) | 11 (22) |
| Very high risk | 17 (85) | 10 (77) | 12 (71) | 39 (78) |
| . | CLL2-BIO . | CLL2-BIG . | CLL2-BAG . | Total . |
|---|---|---|---|---|
| Analysis population (patients with del(17p)/TP53 mut | 21 | 13 | 17 | 51 |
| Age, y | ||||
| Median (range) | 68 (49-81) | 67 (55-81) | 56 (42-73) | 64 (42-81) |
| Patients aged >65 (%) | 12 (57) | 7 (54) | 3 (18) | 22 (43) |
| Sex | ||||
| Male patients (%) | 14 (67) | 6 (46) | 12 (71) | 32 (63) |
| ECOG performance status 1 or 2 (%) | 12 (57) | 5 (38) | 7 (41) | 24 (47) |
| CIRS comorbidity score | ||||
| Median (range) | 4 (1-9) | 4 (0-10) | 2 (0-14) | 3 (0-14) |
| Patients with CIRS >6 (%) | 4 (19) | 3 (23) | 1 (6) | 8 (16) |
| Creatinine clearance | ||||
| Median, mL/min | 80.5 | 77.8 | 79.9 | 79.9 |
| Patients with creatinine clearance <70 mL/min (%) | 6 (29) | 4 (31) | 5 (29) | 15 (29) |
| Previous therapies | ||||
| Patients with ≥1 prior therapy (%) | 13 (62) | 9 (69) | 11 (65) | 33 (65) |
| Patients with prior treatment, median (range) | 2 (1-5) | 1 (1-5) | 3 (1-9) | 2 (1-9) |
| Binet stage (%) | ||||
| A | 5 (24) | 2 (15) | 7 (41) | 14 (28) |
| B | 8 (38) | 2 (15) | 3 (18) | 13 (26) |
| C | 8 (38) | 9 (69) | 7 (41) | 24 (47) |
| Presence of B symptoms (%) | 9 (43) | 3 (23) | 8 (47) | 20 (39) |
| Serum parameters (%) | ||||
| Thymidine kinase >10 U/L | 19 (95) | 12 (92) | 16 (94) | 47 (94) |
| β2-microglobulin >3.5 mg/L | 13 (62) | 7 (54) | 9 (53) | 29 (58) |
| FISH cytogenetics, Döhner model (%) | ||||
| del(17p) | 12 (57) | 8 (62) | 11 (65) | 31 (61) |
| del(11q) | 2 (10) | 0 | 2 (12) | 4 (8) |
| Trisomy 12 | 0 | 0 | 1 (6) | 1 (2) |
| Normal | 4 (19) | 3 (23) | 2 (12) | 9 (18) |
| del(13q) | 3 (14) | 2 (15) | 1 (6) | 6 (12) |
| Molecular genetics (%) | ||||
| IGHV unmutated | 14 (67) | 9 (69) | 15 (88) | 38 (75) |
| TP53 mutated | 20 (95) | 12 (92) | 17 (100) | 49 (96) |
| NOTCH1 mutated | 2 (10) | 2 (15) | 2 (12) | 6 (12) |
| SF3B1 mutated | 4 (19) | 2 (15) | 3 (18) | 9 (18) |
| Complex karyotype, ≥3 aberrations (%) | 13 (68)* | NK* | 10 (59) | 23 (64)* |
| CLL-IPI (%) | ||||
| High | 3 (15) | 3 (23) | 5 (29) | 11 (22) |
| Very high risk | 17 (85) | 10 (77) | 12 (71) | 39 (78) |
CIRS, Cumulative Illness Rating Scale; CLL-IPI, International Prognostic Index for CLL; ECOG, Eastern Cooperative Oncology Group; NK, not known.
Missing patients: 2 in CLL2-BIO and all 13 in CLL2-BIG.
Almost two-thirds of patients (33 of 51) were previously treated with a median of 2 prior regimens (range, 1-9); the most common were chemoimmunotherapies (bendamustine/rituximab in 21 patients and fludarabine/cyclophosphamide/rituximab in 8 patients). Seven patients (14%) had already received a targeted agent (4 therapies contained ibrutinib, 3 idelalisib, and 2 venetoclax) and 2 patients had undergone an allogeneic stem cell transplantation.
Twenty-seven patients (53%; 15 with previously untreated and 12 with relapsed/refractory CLL) started their study treatment with a debulking with up to 2 cycles of bendamustine. All 51 patients received 6 cycles of induction treatment: 21 patients (41%) were treated with IO in the CLL2-BIO trial, 13 (26%) with IG in CLL2-BIG, and 17 (33%) with AG in CLL2-BAG. All but 3 patients continued the same combination in a maintenance phase; 21 of 48 patients (44%) received the full 24 months as planned and 10 of them continued treatment outside of the study after completion of the 24 months maintenance . Seventeen of 48 patients (35%) were able to stop maintenance treatment as per protocol due to a horizontal level; further details on these patients are given in the last paragraph of this section. Seven patients (15%) experienced disease progression during maintenance treatment (CLL2-BIO, 4 of 19 patients [21%]; CLL2-BIG, 1 of 13 patients [8%]; CLL2-BAG, 2 of 16 patients [13%]), including 1 diagnosis of Richter transformation, 2 patients (4%) who stopped due to adverse events, and 1 patient who died related to treatment (2%). The mean duration of targeted treatment was longest in CLL2-BIO (24 months), followed by CLL2-BIG (22 months), and shortest in CLL2-BAG (17 months) (Table 3).
Treatment exposure
| . | CLL2-BIO . | CLL2-BIG . | CLL2-BAG . | Total . |
|---|---|---|---|---|
| Analysis population [patients with del(17p)/TP53 mut] | 21 | 13 | 17 | 51 |
| Debulking | ||||
| Patients with 1-2 cycles bendamustine (%) | 11 (52) | 8 (62) | 8 (47) | 27 (53) |
| Induction | ||||
| Patients with 6 induction cycles with IO, IG, or AG (%) | 21 (100) | 13 (100) | 17 (100) | 51 (100) |
| Early discontinuation at the end/after induction (%) | 2 (10) | 0 | 1 (6) | 3 (6) |
| Maintenance | ||||
| Patients with ≥1 maintenance cycle with IO, IG, or AG (%) | 19 (91) | 13 (100) | 16 (94) | 48 (94) |
| Mean no. of cycles | 6.9 | 4.9 | 3.7 | 5.3 |
| Patients with full 8 maintenance cycles, 24 mo (%) | 13 (68) | 6 (46) | 2 (13) | 21 (44) |
| Early discontinuation of maintenance (%) | ||||
| Due to adverse events | 1 (5) | 1 (8) | 0 | 2 (4) |
| Due to progressive disease | 4 (21) | 1 (8) | 2 (13) | 7 (15) |
| Death | 1 (5) | 0 | 0 | 1 (2) |
| Discontinuation of maintenance due to uMRD (%) | 0 | 5 (39) | 12 (75) | 17 (35) |
| Mean no. (range) of cycles | NA | 2.4 (2-4) | 3.3 (2-6) | 3.0 (2-6) |
| Duration of treatment | ||||
| Mean (range) duration, mo, of | ||||
| All treatment: debulking, induction, maintenance | 25 (5-32) | 23 (13-34) | 18 (4-30) | 22 (4-34) |
| Targeted treatment: induction and maintenance | 24 (5-31) | 22 (12-32) | 17 (4-30) | 21 (4-32) |
| . | CLL2-BIO . | CLL2-BIG . | CLL2-BAG . | Total . |
|---|---|---|---|---|
| Analysis population [patients with del(17p)/TP53 mut] | 21 | 13 | 17 | 51 |
| Debulking | ||||
| Patients with 1-2 cycles bendamustine (%) | 11 (52) | 8 (62) | 8 (47) | 27 (53) |
| Induction | ||||
| Patients with 6 induction cycles with IO, IG, or AG (%) | 21 (100) | 13 (100) | 17 (100) | 51 (100) |
| Early discontinuation at the end/after induction (%) | 2 (10) | 0 | 1 (6) | 3 (6) |
| Maintenance | ||||
| Patients with ≥1 maintenance cycle with IO, IG, or AG (%) | 19 (91) | 13 (100) | 16 (94) | 48 (94) |
| Mean no. of cycles | 6.9 | 4.9 | 3.7 | 5.3 |
| Patients with full 8 maintenance cycles, 24 mo (%) | 13 (68) | 6 (46) | 2 (13) | 21 (44) |
| Early discontinuation of maintenance (%) | ||||
| Due to adverse events | 1 (5) | 1 (8) | 0 | 2 (4) |
| Due to progressive disease | 4 (21) | 1 (8) | 2 (13) | 7 (15) |
| Death | 1 (5) | 0 | 0 | 1 (2) |
| Discontinuation of maintenance due to uMRD (%) | 0 | 5 (39) | 12 (75) | 17 (35) |
| Mean no. (range) of cycles | NA | 2.4 (2-4) | 3.3 (2-6) | 3.0 (2-6) |
| Duration of treatment | ||||
| Mean (range) duration, mo, of | ||||
| All treatment: debulking, induction, maintenance | 25 (5-32) | 23 (13-34) | 18 (4-30) | 22 (4-34) |
| Targeted treatment: induction and maintenance | 24 (5-31) | 22 (12-32) | 17 (4-30) | 21 (4-32) |
After bendamustine debulking, 12 of 27 patients (44%) achieved a partial response (6 each from the previously untreated and relapsed/refractory stratum, ie, 40% and 50% of patients, respectively). Two (1 each with previously untreated and with relapsed/refractory CLL) progressed during debulking but continued with the induction treatment (Table 4). At final restaging after 8 months of induction treatment, 17 patients treated with IO responded (ORR, 81%), 13 patients with IG (100%), and 16 patients with AG (94%). uMRD was achieved in 0, 3, and 14 patients, respectively (0%, 23%, and 82%). Twelve months after start of treatment, 37% of patients had achieved uMRD levels; these rates increased to 49% and 52% after 24 and 36 months (supplemental Figure 2). In addition to the 7 progressions during maintenance (mentioned in the previous paragraph), the MRD level converted from undetectable to intermediate or positive until the end of maintenance treatment in 4 patients (all in the CLL2-BAG trial). In contrast, the continued maintenance treatment led to an improvement of the MRD level from intermediate/positive to undetectable in 7 patients and from positive to intermediate in 10 patients (all in CLL2-BIO and CLL2-BIG; supplemental Table 1a-d). At the end of the maintenance treatment, 14 patients treated with IO responded (ORR, 74%), 11 patients treated with IG responded (85%), and 14 patients treated with AG responded (88%). The corresponding number of patients with a uMRD level was 3 (16%), 6 (46%), and 10 (63%), respectively.
Response to treatment
| . | CLL2-BIO . | CLL2-BIG . | CLL2-BAG . | Total . |
|---|---|---|---|---|
| Debulking, no. of patients (%) | 11 | 8 | 8 | 27 |
| iwCLL response | ||||
| PR | 6 (55) | 2 (25) | 4 (50) | 12 (44) |
| SD | 5 (46) | 3 (38) | 3 (38) | 11 (41) |
| PD | 0 | 2 (25) | 0 | 2 (7) |
| Missing | 0 | 1 (13) | 1 (13) | 2 (7) |
| Induction, no. of patients (%) | 21 | 13 | 17 | 51 |
| iwCLL response | ||||
| CR | 0 | 0 | 1 (6) | 1 (2) |
| PR | 16 (76) | 13 (100) | 15 (88) | 44 (86) |
| PR-L | 1 (5) | 0 | 0 | 1 (2) |
| SD | 3 (14) | 0 | 0 | 3 (6) |
| PD | 1 (5) | 0 | 1 (6) | 2 (4) |
| MRD response: PB | ||||
| Undetectable/low, <10−4 | 0 | 3 (23) | 14 (82) | 17 (33) |
| Intermediate, ≥10−4 and <10−2 | 2 (10) | 5 (39) | 2 (12) | 9 (18) |
| Positive, ≥10−2 | 18 (86) | 4 (31) | 0 | 22 (43) |
| Missing | 1 (5) | 1 (8) | 1 (6) | 3 (6) |
| Maintenance, no. of patients (%) | 19 | 13 | 16 | 48 |
| iwCLL response | ||||
| CR | 0 | 0 | 5 (31) | 5 (10) |
| PR | 14 (74) | 11 (85) | 9 (56) | 34 (71) |
| PD | 5 (26) | 1 (8) | 2 (13) | 8 (17) |
| Missing | 0 | 1 (8) | 0 | 1 (2) |
| MRD response: PB* | ||||
| Undetectable/low, <10−4 | 3 (16) | 6 (46) | 10 (63) | 19 (40) |
| Intermediate, ≥10−4 and <10−2 | 7 (37) | 4 (31) | 2 (13) | 13 (27) |
| Positive, ≥10−2 | 9 (47) | 0 | 4 (25) | 13 (27) |
| Missing | 0 | 3 (23) | 0 | 3 (6) |
| MRD response: BM† | ||||
| Undetectable/low, <10−4 | 0 (0.0) | 2 (15.4) | 6 (37.5) | 8 (16.7) |
| Intermediate, ≥10−4 and <10−2 | 1 (5.3) | 1 (7.7) | 2 (12.5) | 4 (8.3) |
| Positive, ≥10−2 | 2 (10.5) | 0 (0.0) | 0 (0.0) | 2 (4.2) |
| Missing | 16 (84.2) | 10 (76.9) | 8 (50.0) | 34 (70.8) |
| . | CLL2-BIO . | CLL2-BIG . | CLL2-BAG . | Total . |
|---|---|---|---|---|
| Debulking, no. of patients (%) | 11 | 8 | 8 | 27 |
| iwCLL response | ||||
| PR | 6 (55) | 2 (25) | 4 (50) | 12 (44) |
| SD | 5 (46) | 3 (38) | 3 (38) | 11 (41) |
| PD | 0 | 2 (25) | 0 | 2 (7) |
| Missing | 0 | 1 (13) | 1 (13) | 2 (7) |
| Induction, no. of patients (%) | 21 | 13 | 17 | 51 |
| iwCLL response | ||||
| CR | 0 | 0 | 1 (6) | 1 (2) |
| PR | 16 (76) | 13 (100) | 15 (88) | 44 (86) |
| PR-L | 1 (5) | 0 | 0 | 1 (2) |
| SD | 3 (14) | 0 | 0 | 3 (6) |
| PD | 1 (5) | 0 | 1 (6) | 2 (4) |
| MRD response: PB | ||||
| Undetectable/low, <10−4 | 0 | 3 (23) | 14 (82) | 17 (33) |
| Intermediate, ≥10−4 and <10−2 | 2 (10) | 5 (39) | 2 (12) | 9 (18) |
| Positive, ≥10−2 | 18 (86) | 4 (31) | 0 | 22 (43) |
| Missing | 1 (5) | 1 (8) | 1 (6) | 3 (6) |
| Maintenance, no. of patients (%) | 19 | 13 | 16 | 48 |
| iwCLL response | ||||
| CR | 0 | 0 | 5 (31) | 5 (10) |
| PR | 14 (74) | 11 (85) | 9 (56) | 34 (71) |
| PD | 5 (26) | 1 (8) | 2 (13) | 8 (17) |
| Missing | 0 | 1 (8) | 0 | 1 (2) |
| MRD response: PB* | ||||
| Undetectable/low, <10−4 | 3 (16) | 6 (46) | 10 (63) | 19 (40) |
| Intermediate, ≥10−4 and <10−2 | 7 (37) | 4 (31) | 2 (13) | 13 (27) |
| Positive, ≥10−2 | 9 (47) | 0 | 4 (25) | 13 (27) |
| Missing | 0 | 3 (23) | 0 | 3 (6) |
| MRD response: BM† | ||||
| Undetectable/low, <10−4 | 0 (0.0) | 2 (15.4) | 6 (37.5) | 8 (16.7) |
| Intermediate, ≥10−4 and <10−2 | 1 (5.3) | 1 (7.7) | 2 (12.5) | 4 (8.3) |
| Positive, ≥10−2 | 2 (10.5) | 0 (0.0) | 0 (0.0) | 2 (4.2) |
| Missing | 16 (84.2) | 10 (76.9) | 8 (50.0) | 34 (70.8) |
BM, bone marrow; PB, peripheral blood; PD, progressive disease; PR, partial remission; PR-L, partial remission with lymphocytosis; SD, stable disease.
Last staging during maintenance (up to 3 months before termination of treatment).
MRD assessment at any time point between final restaging at the end induction and end of maintenance treatment.
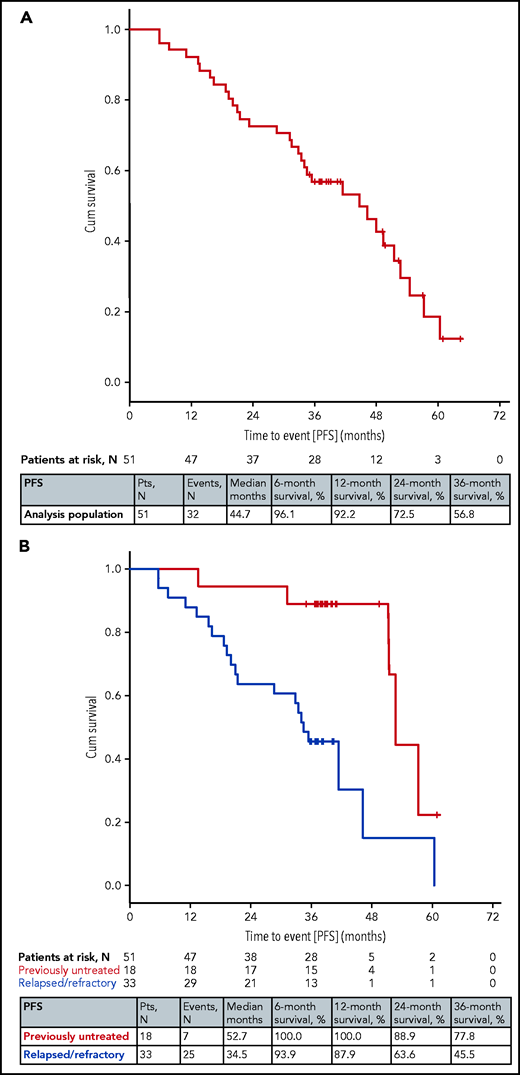
After a median observation time of 49 months (range, 8-68 months), 32 events for progression-free survival (PFS) were documented, including 4 deaths without progression. Five of the 28 progressions were due to Richter transformation (3 occurred in CLL2-BAG and 1 each in CLL2-BIG and CLL2-BIO). The median PFS was 45 months and the rates at 12, 24, and 36 months were 92%, 73%, and 57% (Figure 1A). Seven PFS events occurred in the previously untreated patients (7 of 18; 39%), the median PFS was 53 months with 24- and 36-month PFS rates of 89% and 78%, respectively. Twenty-five of 33 patients (76%) with relapsed/refractory disease progressed or died; the median PFS was 35 months with 24- and 36-month rates of 64% and 46% (Figure 1B). No differences in PFS were seen for the 3 subgroups with different therapies (supplemental Figure 3). The efficacy outcomes of patients with mutated and unmutated IGHV status showed no relevant differences (supplemental Table 2).
PFS. (A) PFS in all 51 patients. (B) PFS by prior treatment (previously untreated and relapsed/refractory). Cum, cumulative.
PFS. (A) PFS in all 51 patients. (B) PFS by prior treatment (previously untreated and relapsed/refractory). Cum, cumulative.
Genes recurrently mutated in CLL were analyzed at baseline and first progression in 8 patients with relapsed/refractory disease (5 from CLL2-BIO and 3 from CLL2-BAG); for the 20 other patients with a progression, no materials, or only samples with insufficient quality or tumor cell quantity, were available. The changes including the variant allele fraction of TP53 and the fraction of del(17p) cells are depicted in supplemental Figure 5. Two patients acquired a BTK C481S mutation (ibrutinib-resistance mutation); both had received ibrutinib over the whole duration of maintenance and progressed during or within 3 months after end of treatment, respectively. No mutations in BCL2 were detected.
Ten patients (7 from CLL2-BIO and 3 from CLL2-BIG) continued treatment with ibrutinib beyond the completion of the planned 24 months of maintenance treatment (2 patients from CLL2-BIG in combination with obinutuzumab). Twenty patients received subsequent therapies after progression, among them 14 with a targeted agent (mainly venetoclax or ibrutinib), 3 with an intense chemo(immuno)therapy with rituximab with cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP), and 2 with a chemoimmunotherapy.
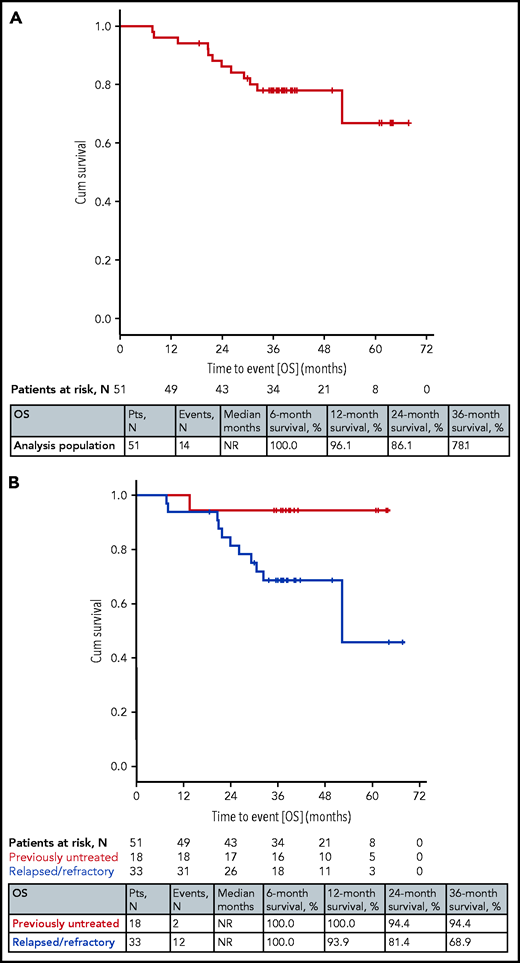
Fourteen patients died (all but 2 in the relapsed/refractory stratum); the median overall survival (OS) was not reached and the rates at 12, 24, and 36 months were 96%, 86%, and 78% (Figure 2). Nine patients died due to a progression of CLL; 3 of them were diagnosed with a Richter transformation (1 received chimeric antigen receptor T cells and died of a sepsis). Three patients died due to infections: 2 during CLL2-BIO study treatment (1 sepsis and 1 ischemic cerebral infarction in the context of an infectious complication and atrial fibrillation) and 1 from CLL2-BIG during subsequent treatment. Furthermore, 1 patient died due to a ventricular arrhythmia and heart failure during subsequent treatment with ibrutinib and for 1 patient no reason for death was documented in the registry. No notable differences were seen with the different therapies (supplemental Figure 4).
OS. (A) OS in all 51 patients. (B) OS by prior treatment (previously untreated and relapsed/refractory).
OS. (A) OS in all 51 patients. (B) OS by prior treatment (previously untreated and relapsed/refractory).
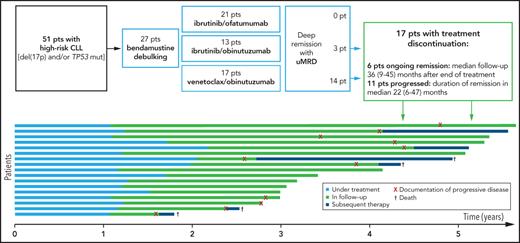
Seventeen patients (33%) with a deep remission with uMRD stopped maintenance treatment as per protocol, among them 6 with previously untreated and 11 with relapsed/refractory CLL (12 from the CLL2-BAG and 5 from the CLL2-BIG study; see baseline characteristics in supplemental Table 3). These patients stopped treatment after a mean of 3 maintenance cycles (range, 2-6 cycles), that is in total, ∼17 months of targeted treatment. Nine of the 17 patients (53%) had a clinical relapse (including 3 with a Richter transformation) and 2 patients died without progression. All but 2 PFS events occurred in the CLL2-BAG trial (7 patients) and all except 1 in the relapsed/refractory stratum (8 patients). The median PFS after discontinuation of treatment was 28 months. Relapse therapies were venetoclax (single agent or combinations) in 4 patients, intense chemoimmunotherapies with R-CHOP/rituximab with dexamethasone, cytarabine, and cisplatin (in 2 patients), and investigational therapies (1 patient). Four patients died, 1 of progressive CLL and 2 of an infectious complication in the context of the relapse therapy with R-CHOP for Richter transformation; for 1 patient, no further information was documented. After a median observation time of 46 months (range, 6-47 months) after discontinuation of treatment, 6 of the 17 patients (35%) are still in remission (Figure 3).
Swimmers plot of 17 patients with termination of treatment due to uMRD. Length of the bar shows duration of treatment (red), observation after treatment discontinuation (green) and start of next treatment line (dark blue). The time point of first documentation of uMRD in peripheral blood and bone marrow is indicated with a circle and a black dot, respectively. Documentation of progressive disease, Richter transformation, and death are as described in the symbol key in the image. BM, bone marrow; PB, peripheral blood.
Swimmers plot of 17 patients with termination of treatment due to uMRD. Length of the bar shows duration of treatment (red), observation after treatment discontinuation (green) and start of next treatment line (dark blue). The time point of first documentation of uMRD in peripheral blood and bone marrow is indicated with a circle and a black dot, respectively. Documentation of progressive disease, Richter transformation, and death are as described in the symbol key in the image. BM, bone marrow; PB, peripheral blood.
Discussion
This pooled analysis of 51 patients with CLL harboring del(17p) and/or TP53 mut from 3 phase 2 trials showed that the vast majority of patients with these high-risk genetic alterations responded to the combination of IO or IG, or AG. One-half of the patients achieved uMRD levels in peripheral blood at some time point. One-third of the patients stopped treatment per protocol after a median of 17 months of targeted treatment because they achieved a deep remission with uMRD levels. In a high proportion of these cases, the remissions were durable: the 17 patients with early discontinuation per protocol due to uMRD had a median PFS of ∼2 years (28 months) after discontinuation of treatment, 9 patients progressed and 2 died without progression. After a median observation time of almost 4 years (46 months; range, 6-47 months) after discontinuation of treatment, 6 patients remained in remission.
In the entire population of high-risk patients, 7 of 18 patients (39%) with previously untreated CLL and 25 of 33 patients (76%) with relapsed/refractory disease progressed or died, the median PFS was 53 vs 35 months, respectively, and the 2- and 3-year rates were 89% and 78% vs 64% and 46%, respectively. Fourteen patients died (all but 2 with relapsed/refractory CLL); the median OS was not reached in both the first-line and relapsed/refractory stratum. The 2- and 3-year PFS rates in the first-line cohort are comparable with those reported by Ahn et al in a subgroup analysis of 34 patients with previously untreated CLL with a del(17p)/TP53 mut who received continuous ibrutinib in a phase 2 trial. Earlier, the authors reported a 2-year PFS of 85% and recently 6-year rates of 61% for PFS and 79% for OS.14,30 An analysis of all patients included in that trial demonstrated that previous treatment significantly affected the outcome of patients with del(17p)/TP53 mut, as the estimated 5-year PFS and OS was 74% and 85% in treatment-naive patients but 19% and 54% in previously treated patients.30 Although the follow-up is shorter in our trials, this difference by prior treatment is also seen, and the fact that two-thirds of the patients had relapsed/refractory CLL, with some being extensively pretreated, including some also with a targeted agent, explains the somewhat worse outcomes in our trials compared with those with previously untreated patients only.
The IGHV status appeared to have no additional impact on the outcome of these high-risk patients treated with targeted agents, as also observed in the CLL14 trial for patients treated with venetoclax/obinutumab.31 However, the relapsed/refractory disease status seems to have a negative impact on the survival times. Nevertheless, the PFS and OS times of all patients and especially of the previously untreated patients are very favorable when considering that all patients included in this analysis have a del(17p)/TP53 mut and several other adverse risk factors were enriched in this cohort, for example, two-thirds with an unmutated IGHV status and a relevant proportion with a complex karyotype.
The interpretation of the clinical data of this analysis is somewhat limited by the different therapies applied because the trials evaluated 3 different combination therapies and one-half of the patients received a debulking with bendamustine ahead of these combinations. In addition, the patient cohort was heterogeneous, as the trials included first-line and relapsed/refractory patients irrespective of type of prior therapy, presence of CLL-associated risk factors as well as physical fitness and comorbidities. However, it can also be argued that this better reflects reality and helps to transfer the results to clinical routine. Another limitation is the low rate of MRD assessments from bone marrow and confirmed CRs due to missing bone marrow biopsies and/or computed tomography scans. Also, the use of additional follow-up data from a registry in 21 of the 51 patients may be criticized because there might be a bias in reporting (eg, preferential inclusion and documentation of patients with a more favorable course of disease). Nevertheless, this is the first report on a longer follow-up after discontinuation of targeted treatment in patients with high-risk genetic alterations who achieved uMRD levels.
Cross-trial comparisons need to be interpreted with caution; the 1 presented in this manuscript might be confounded by the different therapeutic modalities, imbalances in patient characteristics and observation times, as well as small patient numbers. Nevertheless, the data suggest that the 3 combinations appear to vary in efficacy and response dynamics. Although the ORRs were comparable, the rates of uMRD showed relevant differences at the end of the induction phase: none of the patients treated with IO, 3 of 13 (23%) treated with IG, and 14 of 17 patients (82%) treated with AG achieved uMRD levels. These differences were not equalized by continued therapy until the end of the maintenance treatment, but almost one-half of the high-risk patients treated with IG and a few of those treated with IO achieved uMRD levels, which is rarely seen with single-agent ibrutinib.3-932 Interestingly, in CLL2-BAG, none of the 3 patients who did not achieve uMRD levels until the end of the induction phase showed an improvement with continued AG maintenance, whereas in CLL2-BIG and CLL2-BIO, 17 patients improved their MRD level with continued maintenance with IG or IO. The observation that AG induced faster remissions in a higher proportion of patients but no further improvement seen after the induction phase is in line with the results from the CLL14 trial in which most of the patients in the AG arm already had uMRD levels after 6 months of treatment and only 25% showed an improvement thereafter.33 The 2 ibrutinib-containing regimens showed low rates of uMRD early during treatment, which then increased during the maintenance phase; this deepening of response with continued treatment is consistent with the increasing CR rate seen in ibrutinib trials.4,6
As expected, the treatment outcomes in this subgroup analysis of patients with high-risk disease are worse than those reported for the entire patient collective of the 3 trials. The differences described herein for the 3 different regimens were seen in the subgroup of patients with del(17p)/TP53 mut and similarly in the entire patient population of the trials.23-25 No clear differences in PFS or OS can be seen for the 3 therapeutic regimens.
The addition of an anti-CD20 antibody to ibrutinib is a controversial issue. Two randomized trials demonstrated that the addition of rituximab to ibrutinib induces faster and deeper remissions, but did not improve PFS or OS.7,32 However, a randomized 3-arm trial comparing the other BTK inhibitor acalabrutinib with or without obinutuzumab to chemoimmunotherapy showed a nonsignificant difference in PFS in favor of acalabrutinib with obinutuzumab vs single-agent acalabrutinib (24-months rates of 93% vs 87%).34 The choice of the more potent antibody obinutuzumab, and the sample size, might play a role; furthermore, in the context of a treatment that aims at deep remissions to allow for a discontinuation of treatment, the depth of response is certainly more important than in the context of a continuous therapy. Although no samples were available for patients treated with IG, it was shown in 2 patients that the addition of ofatumumab to ibrutinib did not prevent the development of a BTK C481S-resistance mutation. Thus, the role of obinutuzumab in combination with ibrutinib, and especially if used to enable treatment discontinuations, requires further investigation, whereas its role together with venetoclax as a time-limited treatment seems undisputed.2,35
Although the presence of a del(17p)/TP53 mut is linked to resistance to chemotherapy because these agents mediate cell death through DNA damage and p53-dependent apoptosis,36,37 the trial protocols also recommended a bendamustine debulking for patients with these high-risk genetic abnormalities if they had a higher tumor load and no contraindications for the use of bendamustine. The goal of the bendamustine debulking was to reduce the tumor load prior to the targeted treatment, and to decrease the risk of infusion-related reactions and tumor lysis syndromes. Preliminary results of a pooled analysis including all patients treated in these 3 trials showed that the majority of patients experienced adverse events during the debulking, but the overall rates of adverse events and especially of cytopenias and infections were not increased whereas the rate of infusion-related reactions was lower in patients with prior bendamustine compared with those without.38 Considering that the goal of the debulking was to reduce the lymphocyte counts and lymph node size, the finding that 12 of the 27 patients with del(17p)/TP53 mut even achieved a partial response according to iwCLL criteria after only 2 cycles of bendamustine debulking is more than expected in this high-risk cohort. Thus, the use of a short course of chemotherapy may also be a valid approach in patients with high-risk genetic alterations to reduce the tumor load (and hence also potentially the genetic heterogeneity of the tumor) before initiating a targeted treatment, for example, with venetoclax in the outpatient setting. Further analyses are under way.
Thirteen patients had to discontinue treatment early, 3 at the end of the induction phase and 10 during the maintenance treatment. In the majority of 9 patients, this was due to a progression of CLL (including 1 Richter transformation) and/or continuation with a different treatment, which is explained by the risk factors and prior treatments of these patients. Four patients had to stop study treatment because of adverse events, including 2 with fatal complications. With 2 fatalities in 51 patients, the treatment-related mortality is 4% and in an expected range. Four discontinuations due to adverse events and none because of patient wish/withdrawal of consent is rather low compared with other trials with novel agents.3-5,7-9,12,39,40 The good treatment adherence might be explained by more highly motivated patients because of the threat posed by the high-risk genetic alterations as well as the perspective that treatment will be stopped if possible.
Twenty-one patients completed the 24 months of maintenance treatment and 10 of these patients continued treatment thereafter outside of the trial. This continuation of treatment was permitted by protocol because ibrutinib is established as an indefinite treatment, and patients with residual CLL cells should be offered to continue treatment as in routine clinical practice. Unfortunately, efficacy data regarding the continuation of maintenance therapy and regarding the next treatment line after progression is incomplete. Furthermore, the numbers of patients with subsequent therapies in each of the 3 trials is low. Therefore, it is not possible to draw any conclusion on the repetition of the same therapy and on the sequence of therapeutic options.
Seventeen patients with uMRD levels and no other evidence of residual disease (some were partial remissions according to iwCLL criteria because they were lacking a confirmation by computed tomography imaging and/or bone marrow biopsy) stopped study treatment as per protocol after a mean of 17 months of targeted treatment. After a median observation time of almost 4 years (range, 6-47 months) after discontinuation of study treatment, 6 patients are still in remission. Nine patients experienced disease progression and 2 patients died without progression. The median PFS after discontinuation of treatment was ∼2 years. These durable remissions were more common in patients with previously untreated (3 of 6; 50%) compared with relapsed/refractory disease (3 of 11; 27%) and in patients treated in the CLL2-BIG trial (3 of 5; 60%) compared with CLL2-BAG (3 of 12; 25%). However, these numbers are small and it needs to be acknowledged that the proportion of high-risk patients in the CLL2-BAG trial was higher than in CLL2-BIG (17 of 63 [27%] vs 13 of 61 [21%]) and more patients from CLL2-BAG were able to discontinue treatment earlier due to uMRD (12 of 17 [71%] vs 5 of 13 [38%]).
An individualized approach that tailors the duration of treatment to the patient’s response and stops treatment in case of a CR with uMRD levels in peripheral blood appears to be feasible as PFS and OS times were very favorable and disease control was durable in a relevant proportion of patients with high-risk CLL over a long time span. When looking at PFS, it is important to note that the occurrence of a progression does not equal a need for treatment initiation; thus, the treatment-free interval for patients is even longer than 2 years (which is also depicted in the swimmer plot; Figure 3). As this analysis shows that patients with the high-risk genetic markers del(17p) and TP53 mut remained in remission for a relevant time after discontinuation of treatment, treatment-free intervals might become an option, especially during first-line therapy. However, it is currently not known how the disease responds to the next treatment line as compared with a continuous treatment. Termination of ibrutinib treatment would overcome the need for an indefinite treatment, which is so far needed to maintain responses with the BTK inhibitors. Shortened treatment durations might improve patient compliance and could avoid resistance due to clonal evolution arising from therapeutic pressure to select small subclones with resistance mechanisms.10,13
Acknowledgments
The authors express their gratitude toward all patients participating in the trial, as well as the physicians and trial staff at the sites and in the German CLL Study Group. No professional medical-writing services were used. The 3 trials are investigator-initiated trials with the University of Cologne being the legal sponsor.
This work was supported by pharmaceutical companies F. Hoffmann-LaRoche, Janssen-Cilag, and Novartis; the study drugs were provided by AbbVie, F. Hoffmann-LaRoche, Janssen-Cilag, and Novartis. E.T. and S.S. were supported by the Deutsche Forschungsgemeinschaft (DFG; SFB1074, subproject B1 and B2).
Authorship
Contribution: P.C., J.v.T., B.E., and M.H. were responsible for the conception and design of the studies; P.C., J.v.T., S.R., M.F., P.L., O.A.-S., A.M.F., and K.F. were responsible for trial management; P.C., J.v.T., E.T., C.S., M.F., P.L., O.A.-S., B.W.P., C.-M.W., B.E., M.K., S.S., and M.H. were responsible for the recruitment and treatment of patients; P.C., J.v.T., A.G., S.R., M.F., and A.M.F. had access to the raw data; P.C., J.v.T., M.F., P.L., and O.A.-S. performed a central review of all clinical data; E.T., C.S., M.K., and S.S. performed the laboratory analyses; A.G. and S.R. performed the statistical analysis; P.C. wrote the first draft of the manuscript; and all authors interpreted the data and reviewed and approved the manuscript.
Conflict-of-interest disclosure: P.C. reports research funding from AbbVie, Acerta, AstraZeneca, BeiGene, F. Hoffmann-LaRoche, Gilead, GlaxoSmithKline, Janssen-Cilag, and Novartis; honoraria for scientific talks from AbbVie, AstraZeneca, F. Hoffmann-LaRoche, and Janssen-Cilag; honoraria for advisory boards from AbbVie, Acerta, AstraZeneca, Janssen-Cilag, and Novartis; and travel grants from AbbVie, F. Hoffmann LaRoche, Gilead, and Janssen-Cilag. E.T. reports travel grants from AbbVie, and honoraria for consultancy or advisory boards and speakers’ bureaus from AbbVie, Janssen-Cilag, and Roche. J.v.T. reports research funding from F. Hoffmann-LaRoche and Janssen-Cilag; honoraria for advisory boards from AbbVie, F. Hoffmann-LaRoche, and Janssen-Cilag; and travel grants from Celgene, F. Hoffmann-LaRoche, and Janssen-Cilag. P.L. reports research funding from AbbVie, F. Hoffmann-LaRoche, and Janssen-Cilag; honoraria from AstraZeneca and Janssen-Cilag; and travel grants from F. Hoffmann-LaRoche, Janssen-Cilag, and Mundipharma. O.A.-S. reports honoraria and personal fees from AbbVie, AstraZeneca, F. Hoffmann-LaRoche, Gilead, and Janssen-Cilag, as well as research support from AbbVie, BeiGene, F. Hoffmann-LaRoche, and Janssen-Cilag/Pharmacyclics. A.M.F. reports research grants from Celgene; advisory board participation for AbbVie; and travel grants from AbbVie and F. Hoffmann-LaRoche. K.F. reports honoraria from AbbVie and F. Hoffmann-LaRoche and travel grants from F. Hoffmann-LaRoche. C.-M.W. reports research support, honoraria for consultancy or advisory board participation, and travel support from AbbVie, AstraZeneca, F. Hoffmann-LaRoche, Genentech, Gilead, GlaxoSmithKline, Janssen-Cilag, Mundipharma, and Pharmacyclics. B.E. reports research support and honoraria for consultancy or advisory board participation from AbbVie, Adaptive, ArQule, BeiGene, Celgene, F. Hoffmann-LaRoche, Gilead, Janssen, Merck Sharp & Dohme (MSD), and Novartis. M.K. reports honoraria for consultancy or advisory board participation from AbbVie and F. Hoffmann-LaRoche. S.S. reports research support, honoraria for consultancy or advisory board participation, and travel support from AbbVie, Amgen, Celgene, F. Hoffmann-LaRoche, Genentech, Gilead, GlaxoSmithKline, Janssen-Cilag, Mundipharma, Novartis, and Pharmacyclics. M.H. reports research support and honoraria from AbbVie, AstraZeneca, Celgene, F. Hoffmann-LaRoche, Gilead, Janssen, and Pharmacyclics. The remaining authors declare no competing financial interests.
Correspondence: Paula Cramer, German CLL Study Group, University Hospital Cologne Department I of Internal Medicine, 50937, Cologne, Germany.; e-mail: paula.cramer@uk-koeln.de.
Requests for original data may be e-mailed to the corresponding author.
The online version of this article contains a data supplement.
There is a Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal