In this issue of Blood, Bolous et al1 analyze the cost-effectiveness of factor IX gene therapy in patients with severe hemophilia B, in a microsimulation Markov model.
Hemophilia is an X-linked disease that affects 400000 people worldwide, of whom 15% have hemophilia B caused by deficiency of factor IX (FIX), which affected the offspring of Queen Victoria, the most famous carrier. In those with severe deficiency (<1% factor IX activity), the disease is characterized by spontaneous and traumatic bleeding, primarily into joints, which leads to chronic arthropathy, disability, and pain. Current management requires factor infusion several times weekly to prevent bleeding episodes (prophylaxis) and early, intense treatment of acute breakthrough bleeding. Unfortunately, the invasiveness of several times weekly IV therapy deters compliance, and the high cost deprives those in resource-poor settings of life-saving treatment.
Clearly, better therapeutic approaches are needed. The emerging novel agents in development for hemophilia provide great promise. Among these, the “one-and-done” concept of gene therapy is most compelling from a compliance perspective as well as from a global perspective. The goal of gene therapy is to avoid bleeding events in joints and other tissues by long-term factor expression, which generally requires a factor level of 15% or higher. In hemophilia gene therapy, adeno-associated virus (AAV), which is nonpathogenic in humans, is used to package the gene of interest, in this case human factor IX (hFIX). The AAV-hFIX transgene is intravenously injected, targeting the hepatocyte where FIX is synthesized, and commandeers the hepatocyte nuclear machinery to express and secrete FIX protein into the circulation. (AAV is to be distinguished from adenovirus [Ad] COVID vaccines).
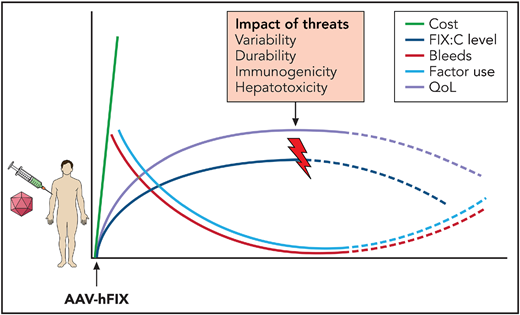
The cost of gene therapy is balanced against the benefits, including reduced bleeding episodes and factor use and improved quality of life, and the potential threats, including the variability and durability of gene expression, immunogenicity, and hepatotoxicity. Professional illustration by Somersault18:24.
The cost of gene therapy is balanced against the benefits, including reduced bleeding episodes and factor use and improved quality of life, and the potential threats, including the variability and durability of gene expression, immunogenicity, and hepatotoxicity. Professional illustration by Somersault18:24.
Currently, there are 2 active clinical gene therapy trials for hemophilia B: the Spark/Pfizer bioengineered vector capsid FIX Padua transgene, fidanacogene elaparvovec (PF-06838435; www.clinicaltrials.gov #NCT038612732), and the UniQure AAV5-FIX Padua construct, etranacogene dezaparvovec (AMT-061; #NCT03569891).3 The FIX-Padua gene, used in each, is a naturally occurring FIX missense mutation associated with eightfold greater specific activity than wild type,4 which allows for lower vector dose and potentially fewer adverse effects. To date, these studies demonstrate >90% reduction in bleeding events and factor use, with sustained factor expression for up to 3 years.
In this setting, Bolous and colleagues employed a microsimulation Markov model to compare the cost of single-dose gene therapy vs the cost of lifelong factor prophylaxis and bleeding complications during on-demand, acute bleeding management, orthopedic surgery, and hospitalization in a sample cohort of 500 000 men ≥18 years of age with severe hemophilia B and no past or current inhibitor. The primary end point was annualized bleeding rate, now preferred over factor levels as a direct measure of hemophilia severity. They assessed microsimulation by probabilistic sensitivity analyses, varying multiple parameters across individual-level disease trajectories, and using literature-based, joint-related disutilities. Estimating the cost of gene therapy at $2 million, the outcome measured in quality-of-life years (QALYs) indicated that hemophilia B gene therapy was more effective than standard factor-based therapy in >90% of simulations, consistent with findings of cost-effectiveness analyses for hemophilia A gene therapy.5 In general, although the price of gene therapy is steep, it is not as costly as factor, orthopedic surgery, bleeding management, and hospitalization over an adult lifetime.
Although these are encouraging findings, much work remains. Novel approaches are needed to adapt current vector-gene technology for those excluded from gene therapy: those with inhibitor alloantibodies, natural AAV antibody,6 and active hepatitis B or hepatitis C liver disease.
Approaches to hepatocyte rescue may be needed in individuals who no longer express the transgene because of concomitant drug toxicity, nonalcoholic steatohepatitis, or viral cirrhosis, which is not uncommon in the hemophilia population.7 Efforts to develop steroid-sparing immunosuppression or vector decoys to reduce the host anti-AAV capsid T-cell response that limits gene expression8 is critical. Finally, careful surveillance is important for determining long-term risks, such as AAV integration and hepatocellular cancer (HCC),9 given the continuing risk for HCC in those with hemophilia10 (see figure).
In summary, it is exciting to consider that after years of complications of hemophilia factor therapy, including inhibitor formation, hepatitis, and HIV, it is now possible to receive a single dose of gene therapy and achieve therapeutic factor levels sufficient to prevent bleeding episodes and avoid factor use and its complications, while providing hope for those globally affected and achieving an improved quality of life.
Conflict-of-interest disclosure: The author declares no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal