In this issue of Blood, Yanatori et al1 demonstrate that the vesicular protein CD63 is regulated by iron and facilitates the secretion of iron-laden ferritin into extracellular vesicles, suggesting that CD63 may enable the transfer of iron-rich ferritin among cells.
Ferritin is a 24-subunit protein nanocage best known for its ability to store intracellular iron in a nontoxic but bioavailable form. A typical ferritin nanocage contains 1000 to 1500 atoms of iron and has the capacity to store >4000 atoms of iron, making it the principal intracellular iron reservoir and a critical contributor to the maintenance of intracellular iron homeostasis. However, ferritin is also found in extracellular compartments, notably human plasma. Levels of ferritin in plasma correlate with body iron stores and are also elevated in inflammation. Clinically, serum ferritin levels are used to assess iron status. The presence of ferritin in extracellular compartments has led to the suspicion that ferritin may play a role in extracellular as well as intracellular spaces, although the nature of that role and the mechanism by which ferritin reaches external environments have remained obscure for decades and are still incompletely understood.
A turning point in our understanding of extracellular ferritin came in 2018, when Truman-Rosentsvit et al2 reported that ferritin can be secreted from macrophages through noncanonical pathways involving extracellular vesicles. However, what triggers ferritin secretion and how it is coordinated with intracellular iron metabolism remained unclear.
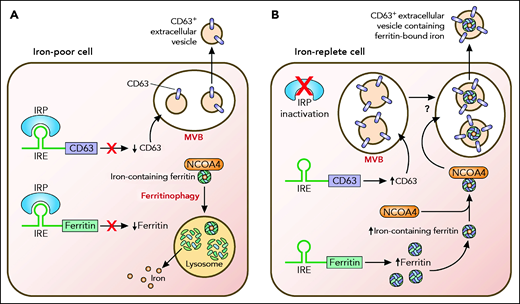
Yanatori et al now demonstrate that increased iron levels increase expression of CD63, a tetraspanin protein and important constituent of extracellular vesicles. Mechanistically, CD63 induction is accomplished through an iron responsive element (IRE) in the 5′ untranslated region of CD63 messenger RNA (mRNA) that is controlled by the same iron regulatory network that controls levels of ferritin itself. This network consists of iron regulatory proteins, which bind to IREs in the untranslated regions of selected mRNAs to posttranscriptionally control their activity or stability (see figure). Targets of the posttranscriptional iron regulatory network include not only ferritin but also proteins that regulate iron uptake and iron efflux, such as transferrin receptor 1 and ferroportin. Thus, the identification of a functional IRE in the 5′ untranslated region of CD63 directly connects CD63 to pathways of iron metabolism.
The authors further show that the induction of CD63 by iron has a functional impact on iron metabolism by increasing the secretion of CD63+ extracellular vesicles containing iron-loaded ferritin. Mechanistically, iron loading stimulates the association of ferritin and its cargo receptor NCOA4 with CD63+ vesicles, facilitating extracellular release of ferritin. Under iron-poor conditions, NCOA4 mediates ferritin degradation to release iron and rebalance intracellular labile iron pools in a process termed ferritinophagy.3 The authors now show that NCOA4 can also mediate ferritin secretion under iron-rich conditions. The mechanism underlying the iron-dependent switch in NCOA4-mediated ferritin trafficking remains unknown. Removal of ferritin-bound iron through CD63+ vesicles is an additional pathway for iron exit from cells that complements the activity of ferroportin, a well-described iron efflux pump that transports elemental iron.
Export of ferritin-bound iron in CD63+ vesicles is enhanced by iron. (A) In an iron-poor cell, translation of CD63 as well as ferritin H and L subunits (represented in the figure as ferritin) is inhibited by iron regulatory proteins (IRPs). Iron-containing ferritin in the cell is directed by the cargo receptor NCOA4 to the lysosome for degradation, a process termed ferritinophagy.3 Ferritinophagy liberates iron contained within ferritin, enabling cellular use of iron for essential processes. (B) In an iron-replete cell, IRPs are inactivated, and translation of both CD63 and ferritin subunits is increased. Ferritin associates with NCOA4 and is directed to CD63+ vesicles by an unclear mechanism. CD63+ vesicles are shown in multivesicular bodies (MVBs) based on work by Truman-Rosentsvit et al2 and Brown et al.4 Ferritin and its associated iron within CD63+ vesicles are ultimately exported out of the cell. Professional illustration by Patrick Lane, ScEYEnce Studios.
Export of ferritin-bound iron in CD63+ vesicles is enhanced by iron. (A) In an iron-poor cell, translation of CD63 as well as ferritin H and L subunits (represented in the figure as ferritin) is inhibited by iron regulatory proteins (IRPs). Iron-containing ferritin in the cell is directed by the cargo receptor NCOA4 to the lysosome for degradation, a process termed ferritinophagy.3 Ferritinophagy liberates iron contained within ferritin, enabling cellular use of iron for essential processes. (B) In an iron-replete cell, IRPs are inactivated, and translation of both CD63 and ferritin subunits is increased. Ferritin associates with NCOA4 and is directed to CD63+ vesicles by an unclear mechanism. CD63+ vesicles are shown in multivesicular bodies (MVBs) based on work by Truman-Rosentsvit et al2 and Brown et al.4 Ferritin and its associated iron within CD63+ vesicles are ultimately exported out of the cell. Professional illustration by Patrick Lane, ScEYEnce Studios.
Collectively, these data are consistent with a model in which induction of CD63 by iron promotes iron removal through secretion of iron-laden ferritin in extracellular vesicles. The authors perform their studies primarily in transformed human fibroblasts and confirm their results in other cell lines, including hepatocellular carcinoma cells and monocytes (HepG2, THP1). This suggests that CD63-mediated secretion of ferritin by extracellular vesicles may be a general pathway of iron export. However, only transformed cells were examined, so further confirmation of this pathway is necessary. This work is consistent with evidence that secretion of ferritin in extracellular vesicles by breast cancer cells reduces their sensitivity to ferroptosis, an iron-dependent form of cell death.4
Mysteries remain. Yanatori et al show that iron promotes the secretion of iron-laden ferritin in extracellular vesicles. What happens to this ferritin and to the iron it contains? One attractive possibility is that it serves as an iron source for neighboring cells, perhaps through binding to ferritin receptors5,6 and catabolism through ferritinophagy. If so, what prevents a futile cycle of ferritin-bound iron secretion and uptake by the same cell? One speculative answer is that in addition to stimulating CD63-dependent secretion of ferritin, iron repletion also discourages uptake of extracellular vesicles, thus redirecting ferritin-bound iron to adjacent, potentially more iron-deficient, neighboring cells. Using radioactive or other tracers to follow the path of both iron and ferritin might be useful in addressing such questions, as would studying this pathway in tissue environments containing multiple cell types.
Another mystery: what is the connection, if any, between ferritin found in extracellular vesicles and serum ferritin? Despite its positive correlation with body iron stores, ferritin found in plasma is notably iron poor7 and therefore different from the iron-rich ferritin identified by Yanatori et al in extracellular vesicles. Do these 2 pools of extracellular ferritin represent separate entities? Or are they produced by the same pathway, but with differing iron content based on the iron status or ferritin subunit composition of the producing cell? Alternatively, is iron-rich ferritin taken up by cells, leaving only its iron-poor relative in the plasma, or is the iron somehow removed from iron-containing ferritin in extracellular vesicles to produce iron-poor serum ferritin? If iron is removed from ferritin, how is this accomplished? Because known pathways of iron removal from ferritin such as ferritinophagy result in degradation of the ferritin protein, they do not offer a plausible mechanism for the generation of iron-poor ferritin in the plasma from iron-rich ferritin in extracellular vesicles. Likely, there are more pathways of ferritin and iron trafficking yet to be discovered.
Conflict-of-interest disclosure: The authors declare no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal