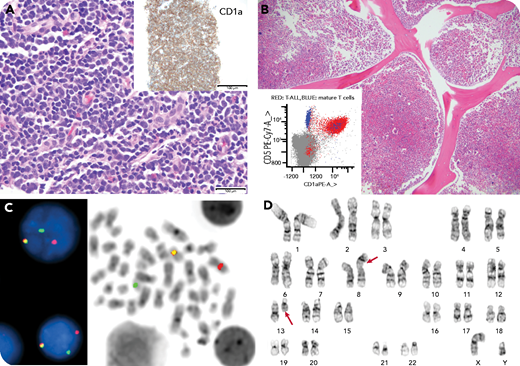
A 25-year-old man presented with a leukocyte count of 174 × 109/L, massive splenomegaly, inguinal lymphadenopathy, night sweats, weight loss, and early satiety for 1 month. The peripheral blood smear revealed marked absolute left-shifted neutrophilia, eosinophilia, and basophilia with flow cytometric detection of 1.3% aberrant myeloblasts and 0.01% immature T cells by minimal residual disease analysis (panel B, inset). The inguinal lymph node biopsy showed diffuse sheets of medium lymphoid cells that stained positive for CD1a and terminal deoxynucleotidyltransferase (panel A, hematoxylin and eosin (H&E) stain, original magnification ×400; inset, original magnification ×100 ). The concurrent flow cytometry analysis confirmed involvement by T-lymphoblastic leukemia/lymphoma. The bone marrow examination showed a hypercellular marrow with left-shifted myeloid and megakaryocytic hyperplasia, with 1% blasts (panel B, H&E stain, original magnification ×100 ). An FGFR1 (8p11) gene rearrangement was detected in the lymph node (85% of cells) and bone marrow (95% of cells) (panel C; break apart FISH with red-31FGFR1, green-51 FGFR1, and yellow fusion signals) by fluorescence in situ hybridization analysis and was negative for BCR-ABL1. Cytogenetics revealed t(8;13) in the bone marrow (panel D).
This is a rare case of an 8p11 myeloproliferative neoplasm that harbors stem cell properties and can present as a bilineage/trilineage neoplasm. There is no definite established remission-induction regimen, and the myeloid neoplasms exhibit chemoresistance to first- and second-generation tyrosine kinase inhibitors (TKIs). Newer TKIs and small molecule FGFR1 inhibitors (pemigatinib; clinical trial FIGHT-203) may be effective bridging therapies to stem cell transplant.
A 25-year-old man presented with a leukocyte count of 174 × 109/L, massive splenomegaly, inguinal lymphadenopathy, night sweats, weight loss, and early satiety for 1 month. The peripheral blood smear revealed marked absolute left-shifted neutrophilia, eosinophilia, and basophilia with flow cytometric detection of 1.3% aberrant myeloblasts and 0.01% immature T cells by minimal residual disease analysis (panel B, inset). The inguinal lymph node biopsy showed diffuse sheets of medium lymphoid cells that stained positive for CD1a and terminal deoxynucleotidyltransferase (panel A, hematoxylin and eosin (H&E) stain, original magnification ×400; inset, original magnification ×100 ). The concurrent flow cytometry analysis confirmed involvement by T-lymphoblastic leukemia/lymphoma. The bone marrow examination showed a hypercellular marrow with left-shifted myeloid and megakaryocytic hyperplasia, with 1% blasts (panel B, H&E stain, original magnification ×100 ). An FGFR1 (8p11) gene rearrangement was detected in the lymph node (85% of cells) and bone marrow (95% of cells) (panel C; break apart FISH with red-31FGFR1, green-51 FGFR1, and yellow fusion signals) by fluorescence in situ hybridization analysis and was negative for BCR-ABL1. Cytogenetics revealed t(8;13) in the bone marrow (panel D).
This is a rare case of an 8p11 myeloproliferative neoplasm that harbors stem cell properties and can present as a bilineage/trilineage neoplasm. There is no definite established remission-induction regimen, and the myeloid neoplasms exhibit chemoresistance to first- and second-generation tyrosine kinase inhibitors (TKIs). Newer TKIs and small molecule FGFR1 inhibitors (pemigatinib; clinical trial FIGHT-203) may be effective bridging therapies to stem cell transplant.
For additional images, visit the ASH Image Bank, a reference and teaching tool that is continually updated with new atlas and case study images. For more information, visit http://imagebank.hematology.org.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal