Key Points
CB2R expression on donor T cells regulates the severity of GVHD and accumulation of proinflammatory CD8+ T cells in target organs.
Therapeutic targeting of the CB2R is dependent upon the pharmacological agonist profile and composition of pathogenic immune cells.
Abstract
Graft-versus-host disease (GVHD) pathophysiology is a complex interplay between cells that comprise the adaptive and innate arms of the immune system. Effective prophylactic strategies are therefore contingent upon approaches that address contributions from both immune cell compartments. In the current study, we examined the role of the type 2 cannabinoid receptor (CB2R), which is expressed on nearly all immune cells, and demonstrated that absence of the CB2R on donor CD4+ or CD8+ T cells or administration of a selective CB2R pharmacological antagonist exacerbated acute GVHD lethality. This was accompanied primarily by the expansion of proinflammatory CD8+ T cells, indicating that constitutive CB2R expression on T cells preferentially regulated CD8+ T-cell alloreactivity. Using a novel CB2ReGFP reporter mouse, we observed significant loss of CB2R expression on T cells, but not macrophages, during acute GVHD, indicative of differential alterations in receptor expression under inflammatory conditions. Therapeutic targeting of the CB2R with the agonists Δ9-tetrahydrocannabinol (THC) and JWH-133 revealed that only THC mitigated lethal T cell–mediated acute GVHD. Conversely, only JWH-133 was effective in a sclerodermatous chronic GVHD model where macrophages contributed to disease biology. In vitro, both THC and JWH-133 induced arrestin recruitment and extracellular regulated kinase phosphorylation via CB2R, but THC had no effect on CB2R-mediated inhibition of adenylyl cyclase. This study shows that the CB2R plays a critical role in the regulation of GVHD and suggests that effective therapeutic targeting is dependent upon agonist signaling characteristics and receptor selectivity in conjunction with the composition of pathogenic immune effector cells.
Introduction
Graft-versus-host disease (GVHD) remains a major cause of morbidity and mortality in patients undergoing allogeneic hematopoietic stem cell transplantation (HSCT). GVHD is classified into 2 phases, termed acute and chronic, which are distinguishable based on temporal characteristics as well as unique clinical and pathological manifestations.1-4 Although T cells are the proximate driver of GVHD, disease progression is the result of the complex interplay between elements of the innate and adaptive arms of the immune system. In fact, it is increasingly being recognized that cells of the innate immune system (eg, macrophages, neutrophils) play pivotal roles in the biology of this disease.5-10 Macrophages, in particular, have been shown to be important in the pathophysiology of chronic GVHD in both murine models and humans.6,7,10 However, despite this evolving mechanistic understanding, a majority of therapeutic strategies for both acute and chronic GVHD have focused on the adaptive immune system, with less regard for approaches that also directly target innate immune cells.
Endocannabinoids are endogenously produced, arachidonic acid-containing bioactive lipids that act widely throughout the body as agonists for several G protein–coupled receptors.11,12 The 2 primary endocannabinoids are N-arachidonoylethanolamine (AEA) and 2-arachidonoyl glycerol (2-AG).11 Both AEA and 2-AG act as agonists at the type 1 cannabinoid receptor (CB1R), which is expressed at high density in the central nervous system and at lower density in metabolic tissues, such as liver and adipose tissue.11 2-AG is also a full agonist of the type 2 cannabinoid receptor (CB2R), which is expressed predominantly by immune cells.13 Increasing evidence demonstrates that the CB2R mediates the effects of the plant-derived cannabinoid Δ9-tetrahydrocannabinol (THC) on the immune system,14 and genetic variants in the CB2R gene (cnr2) have been associated with inflammatory disorders in humans.15-18 Furthermore, the CB2R can regulate cells in both the innate and adaptive arms of the immune system and has specifically been shown to regulate cytokine release19-21 and B- and T-cell differentiation22 and modulate macrophage-mediated inflammation.23-25 Therapeutic targeting of the CB2R has therefore emerged as an area of intense interest for a variety of inflammatory disorders26 ; however, the optimal pharmacological approach remains undefined. The goal of the current study was to delineate the role of CB2R signaling in GVHD pathobiology and determine whether pharmacological targeting of the receptor could ameliorate disease using complementary murine models in which there are pathophysiological contributions from the adaptive and innate arms of the immune system.
Materials and methods
Mice
C57BL/6 (B6) (H-2b), B6.PL (Thy1.1+), Balb/c (H-2d), FVB/N (H-2q), B10.D2 (H-2d), Rag-1−/− (H-2b), and CB2R−/− (B6) mice were bred in the Biomedical Resource Center at the Medical College of Wisconsin, or purchased from The Jackson Laboratory (Bar Harbor, ME). CB2ReGFP reporter mice were constructed by inserting an enhanced green fluorescent gene preceded by an internal ribosomal entry site (IRES) into the 3′ untranslated region of the cnr2 mouse gene.27
Other detailed methods
All other methods are described in the supplemental Data, available on the Blood Web site.
Results
Donor CB2R expression regulates the severity of acute GVHD
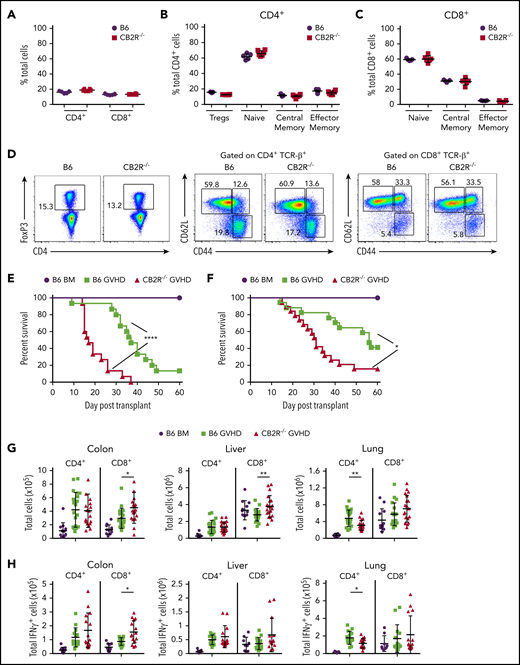
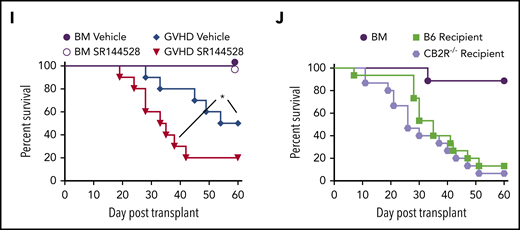
To define the role of CB2R signaling in GVHD biology, we first conducted transplantation studies using CB2R−/− mice as either donors or recipients. We observed that there were no differences in the percentage of total, naïve, central memory, or effector memory splenic CD4+ or CD8+ T cells (Figure 1A-D) or in the frequency of CD4+ Foxp3+ regulatory T cells (Figure 1B,D) between WT B6 and CB2R−/− mice, indicating that the transgenic environment did not result in obvious alterations in the composition of the T-cell compartment. Balb/c recipients reconstituted with CB2R−/− grafts had accelerated GVHD lethality compared with animals receiving WT B6 graft transplants (Figure 1E). Similar results were observed in a second major histocompatibility complex–incompatible model (B6→FVB), indicating that results were not strain specific (Figure 1F). Examination of GVHD target organs revealed that animals receiving BM or spleen cell transplants from CB2R−/− donors had a significant increase in the absolute number of CD8+ T cells in the colon and liver when compared with mice reconstituted with WT grafts (Figure 1G). Conversely, there was no augmentation in CD4+ T-cell numbers in these tissue sites, and there was an overall decrease in the lungs. In addition, mice receiving CB2R−/− graft transplants had increased numbers of CD8+ IFN-γ+ T cells in the colon (Figure 1H), indicating that donor CB2R expression seemed to be important for the regulation of CD8+ T-cell alloreactivity. To account for the fact that the transgenic environment may have still resulted in unappreciated alterations in T-cell development, we used a complementary pharmacological approach to verify that inhibition of CB2R signaling exacerbated GVHD. Animals administered SR144528, which is a selective CB2R antagonist,28 also had significantly greater mortality (Figure 1I), providing confirmation that inhibition of CB2R signaling worsened GVHD. There was no difference in survival between CB2R−/− and WT animals receiving WT grafts (Figure 1J), indicating that only CB2R expression on donor cells regulated GVHD severity.
Absence of donor CB2R expression exacerbates the severity of acute GVHD. (A) Percentage of CD4+ and CD8+ T cells in the spleen of wild-type (WT; n = 5) and CB2R−/− (n = 6) mice. (B-C) Percentage of CD4+ Foxp3+, naïve (CD62Lhi CD44lo), central memory (CD62Lhi CD44hi), and effector memory (CD62Llo CD44hi) CD4+ T cells (B) and naïve, central memory, and effector memory CD8+ T cells (C) in the spleen of WT B6 and CB2R−/− mice. Data are cumulative results from 5 to 6 mice per group and are presented as the mean ± standard deviation. (D) Representative dot plots of CD4+ and CD8+ T-cell populations are shown together with the gating approach that was used for the data in panels B and C. (E) Lethally irradiated (900 cGy) Balb/c recipients received transplants of bone marrow (BM) alone from B6 mice (5 × 106; n = 9) or BM and spleen cells (adjusted to yield an αβ T-cell dose of 0.6 × 106 cells) from B6 (n = 15) or CB2R−/− (n = 15) animals. Overall survival is depicted. Results are from 3 experiments. (F) Lethally irradiated (1100 cGy) FVB mice received transplants of B6 BM alone (n = 9), B6 BM and spleen cells (n = 17), or CB2R−/− BM and spleen cells (n = 19; adjusted to yield an αβ T-cell dose of 0.85 × 106 cells). Overall survival is depicted. Results are from 3 experiments. (G-H) Lethally irradiated Balb/c mice were transplanted with BM and spleen cells (adjusted to yield an αβ+ T-cell dose of 0.6 × 106 to 0.8 × 106) from B6 or CB2R−/− animals. Animals receiving transplants of B6 BM alone served as controls. The absolute numbers of CD4+ and CD8+ T cells in the colon, liver, and lung 14 days posttransplantation are depicted in panel G (n = 12-20 per group), and absolute numbers of CD4+ or CD8+ interferon-γ–positive (IFN-γ+) T cells in the same organs are shown in panel H (n = 9-15 per group). Data are presented as the mean ± SD. Results are from 3 to 4 experiments. (I) Irradiated Balb/c recipients received transplants of B6 BM and spleen cells (adjusted to yield an αβ T-cell dose of 0.8 × 106 cells). Animals were treated with the CB2R antagonist SR144528 (3 mg/kg; n = 10) or an equivalent amount of vehicle (n = 10) for 14 days beginning on the day of transplantation. Balb/c mice receiving transplants of B6 BM alone and then treatment with either vehicle (n = 6) or SR144528 (n = 3) served as controls. Results are from 2 experiments. (J) Lethally irradiated (1100 cGy) B6 (n = 15) or CB2R−/− (n = 15) animals received transplants of Balb/c BM and spleen cells (adjusted to yield an αβ T-cell dose of 5 × 106 to 5.5 × 106 cells). Balb/c mice receiving transplants of B6 BM alone (n = 9) served as controls. Results are from 3 experiments. *P < .05, **P < .01, ****P < .0001. TCR, T-cell receptor.
Absence of donor CB2R expression exacerbates the severity of acute GVHD. (A) Percentage of CD4+ and CD8+ T cells in the spleen of wild-type (WT; n = 5) and CB2R−/− (n = 6) mice. (B-C) Percentage of CD4+ Foxp3+, naïve (CD62Lhi CD44lo), central memory (CD62Lhi CD44hi), and effector memory (CD62Llo CD44hi) CD4+ T cells (B) and naïve, central memory, and effector memory CD8+ T cells (C) in the spleen of WT B6 and CB2R−/− mice. Data are cumulative results from 5 to 6 mice per group and are presented as the mean ± standard deviation. (D) Representative dot plots of CD4+ and CD8+ T-cell populations are shown together with the gating approach that was used for the data in panels B and C. (E) Lethally irradiated (900 cGy) Balb/c recipients received transplants of bone marrow (BM) alone from B6 mice (5 × 106; n = 9) or BM and spleen cells (adjusted to yield an αβ T-cell dose of 0.6 × 106 cells) from B6 (n = 15) or CB2R−/− (n = 15) animals. Overall survival is depicted. Results are from 3 experiments. (F) Lethally irradiated (1100 cGy) FVB mice received transplants of B6 BM alone (n = 9), B6 BM and spleen cells (n = 17), or CB2R−/− BM and spleen cells (n = 19; adjusted to yield an αβ T-cell dose of 0.85 × 106 cells). Overall survival is depicted. Results are from 3 experiments. (G-H) Lethally irradiated Balb/c mice were transplanted with BM and spleen cells (adjusted to yield an αβ+ T-cell dose of 0.6 × 106 to 0.8 × 106) from B6 or CB2R−/− animals. Animals receiving transplants of B6 BM alone served as controls. The absolute numbers of CD4+ and CD8+ T cells in the colon, liver, and lung 14 days posttransplantation are depicted in panel G (n = 12-20 per group), and absolute numbers of CD4+ or CD8+ interferon-γ–positive (IFN-γ+) T cells in the same organs are shown in panel H (n = 9-15 per group). Data are presented as the mean ± SD. Results are from 3 to 4 experiments. (I) Irradiated Balb/c recipients received transplants of B6 BM and spleen cells (adjusted to yield an αβ T-cell dose of 0.8 × 106 cells). Animals were treated with the CB2R antagonist SR144528 (3 mg/kg; n = 10) or an equivalent amount of vehicle (n = 10) for 14 days beginning on the day of transplantation. Balb/c mice receiving transplants of B6 BM alone and then treatment with either vehicle (n = 6) or SR144528 (n = 3) served as controls. Results are from 2 experiments. (J) Lethally irradiated (1100 cGy) B6 (n = 15) or CB2R−/− (n = 15) animals received transplants of Balb/c BM and spleen cells (adjusted to yield an αβ T-cell dose of 5 × 106 to 5.5 × 106 cells). Balb/c mice receiving transplants of B6 BM alone (n = 9) served as controls. Results are from 3 experiments. *P < .05, **P < .01, ****P < .0001. TCR, T-cell receptor.
CB2R expression on CD4+ or CD8+ T cells primarily regulates CD8+ T-cell alloreactivity
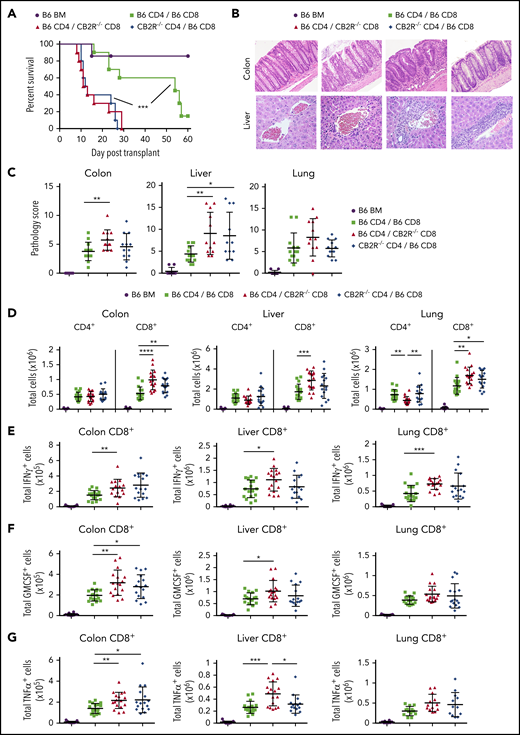
To further interrogate the role of CB2R expression on CD4+ vs CD8+ T cells, we performed transplantations in which animals were reconstituted with mixtures of purified CD4+ and/or CD8+ T cells from either WT or CB2R−/− donors so that the effect of absent CB2R expression in 1 T-cell population could be assessed in the presence of the corresponding WT T-cell population. We observed that absence of the CB2R on either CD4+ or CD8+ T cells resulted in significantly worse survival compared with animals reconstituted with WT CD4+ and WT CD8+ T cells (Figure 2A). Histological analysis revealed that absence of the CB2R on CD8+ T cells exacerbated pathological damage in the colon and liver, whereas transplantation with CD4+ CB2R−/− T cells worsened pathology in the liver compared with WT controls (Figure 2B-C). Examination of GVHD target tissues demonstrated that absence of the CB2R on CD4+ T cells resulted in an increased number of donor CD8+ T cells in the colon and lung, whereas transplantation with CD8+ CB2R−/− T cells led to an increase in the number of CD8+ T cells in the colon, liver, and lung (Figure 2D). Analysis of proinflammatory cytokine production revealed a significantly greater number of CD8+ T cells that produced either IFN-γ, GM-CSF, or TNF-α in the lung, liver, and colon in mice receiving CD8+ CB2R−/− T cell transplants (Figure 2E-G). Notably, absence of the CB2R on CD4+ and/or CD8+ T cells had no effect on the number of CD4+ T cells producing IFN-γ, GM-CSF, or tumor necrosis factor α in any tissue when compared with WT controls (supplemental Figure 1). Thus, CB2R expression primarily on CD8+ T cells was critical for the regulation of CD8+ T-cell expansion and inflammatory cytokine production in GVHD target organs.
Absence of CB2R expression on either CD4+or CD8+T cells exacerbates GVHD mortality. (A-G) Irradiated Balb/c mice received transplants of B6 Rag-1−/− BM (5 × 106; n = 7), Rag-1−/− BM plus purified CD4+ (1 × 106) and CD8+ (0.55 × 106) B6 T cells (n = 10), Rag-1−/− BM plus B6 CD4+ and CD8+ CB2R−/− T cells (n = 10), and Rag-1−/− BM plus CD4+ CB2R−/− and B6 CD8+ T cells (n = 10). Overall survival is shown. Data are from 2 experiments. (B-C) Representative hematoxylin and eosin–stained sections of the lung, liver, and colon of animals 14 days posttransplantation are depicted in panel B. Original magnification is ×100 for photomicrographs. Pathological scores of colon, liver, and lung from mice undergoing transplantation are shown in panel C. Data are from 3 experiments, with 9 to 12 mice per group. (D-G) The absolute numbers of CD4+ and CD8+ T cells in the liver, lung, and colon are shown in panel D. The absolute numbers of CD8+ T cells that produced IFN-γ, granulocyte-macrophage colony-stimulating factor (GM-CSF), or tumor necrosis factor α (TNF-α) in the colon, liver, and lung 14 days posttransplantation are shown in panels E to G. Data are from 4 experiments, with 11 to 17 mice per group. *P < .05, **P < .01, ***P < .001, ****P < .0001.
Absence of CB2R expression on either CD4+or CD8+T cells exacerbates GVHD mortality. (A-G) Irradiated Balb/c mice received transplants of B6 Rag-1−/− BM (5 × 106; n = 7), Rag-1−/− BM plus purified CD4+ (1 × 106) and CD8+ (0.55 × 106) B6 T cells (n = 10), Rag-1−/− BM plus B6 CD4+ and CD8+ CB2R−/− T cells (n = 10), and Rag-1−/− BM plus CD4+ CB2R−/− and B6 CD8+ T cells (n = 10). Overall survival is shown. Data are from 2 experiments. (B-C) Representative hematoxylin and eosin–stained sections of the lung, liver, and colon of animals 14 days posttransplantation are depicted in panel B. Original magnification is ×100 for photomicrographs. Pathological scores of colon, liver, and lung from mice undergoing transplantation are shown in panel C. Data are from 3 experiments, with 9 to 12 mice per group. (D-G) The absolute numbers of CD4+ and CD8+ T cells in the liver, lung, and colon are shown in panel D. The absolute numbers of CD8+ T cells that produced IFN-γ, granulocyte-macrophage colony-stimulating factor (GM-CSF), or tumor necrosis factor α (TNF-α) in the colon, liver, and lung 14 days posttransplantation are shown in panels E to G. Data are from 4 experiments, with 11 to 17 mice per group. *P < .05, **P < .01, ***P < .001, ****P < .0001.
GVHD results in significant loss of CB2R expression on donor T cells
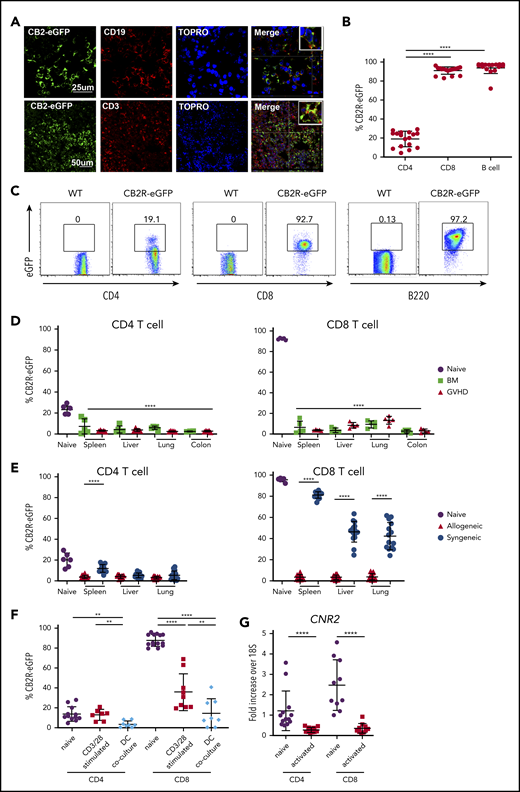
Given the importance of the CB2R in the regulation of GVHD within target organs, we sought to specifically identify receptor-expressing cells in these tissues. A long-standing problem in the field of cannabinoid biology, however, has been the inability to accurately identify CB2R+ cells because of antibody nonspecificity and low receptor expression levels.29 Therefore, we created and used a CB2ReGFP reporter mouse in which expression of eGFP was regulated by CB2R promotor activity through an IRES.27 Immunofluorescent staining of the spleen from these mice demonstrated the presence of eGFP in CD3+ T and CD19+ B cells in merged images, indicative of receptor expression in these populations (Figure 3A). In addition, flow cytometric analysis of splenocytes from CB2ReGFP and WT mice revealed that B cells had the highest expression of the receptor, with nearly all cells (∼97%) expressing eGFP (Figure 3B-C), which is consistent with prior reports using other methods.30 High expression of eGFP was also observed on splenic CD8+ T cells (>90%), whereas expression was significantly lower on CD4+ T cells (∼20%).
GVHD induces a loss of CB2R eGFP expression in T cells isolated from target organs. (A) Spleen sections from CB2ReGFP reporter mice were stained with fluorescence-labeled antibodies directed against eGFP, CD19, or CD3. Topro served as a nuclear stain. Merged results are shown in final panels in each row. (B-C) eGFP expression on CD4+ T cells, CD8+ T cells, and B cells derived from the spleens of normal CB2ReGFP mice (n = 16-17) are shown in panel B. Representative dot plots showing eGFP expression on these immune cell populations are depicted in panel C. (D) Lethally irradiated Balb/c mice received transplants of CB2ReGFP BM alone (n = 4) or together with CB2ReGFP spleen cells (adjusted to yield an αβ+ T-cell dose of 0.6 × 106; n = 5). Animals were euthanized 14 days posttransplantation. The eGFP expression percentage on CD4+ and CD8+ T cells obtained from spleen, liver, lung, and colon of animals receiving transplants of BM alone (BM) or together with adjunctive spleen cells (GVHD) is depicted. The eGFP expression percentage on comparable cells obtained from the spleen of normal nontransplanted CB2ReGFP mice (labeled naïve; n = 5) is shown for comparison. Statistical comparison is from all tissue sites relative to splenic CD4+ and CD8+ T cells from naïve mice. (E) Lethally irradiated Balb/c or B6.PL mice received transplants of CB2ReGFP BM and spleen cells (adjusted to yield an αβ+ T-cell dose of 0.6 × 106). The eGFP expression percentage on CD4+ and CD8+ T cells obtained from spleen, liver, and lung of animals receiving syngeneic vs allogeneic marrow grafts 14 days posttransplantation is depicted. Data are from 3 experiments, with a total of 15 mice per group. The eGFP expression percentage on T cells obtained from the spleen of normal CB2ReGFP mice is shown for comparison. (F) Magnetically purified CD4+ or CD8+ T cells (1 × 105) from CB2ReGFP mice were cultured with anti-CD3 (2.5 μg/mL) and anti-CD28 (5 μg/mL) antibody for 3 days. Magnetically purified pan T cells (1 × 105) from CB2ReGFP mice were cultured with allogeneic Balb/c CD11c–enriched dendritic cells (5 × 104) for 4 days. Data depict the percentage of CD4+ T cells and CD8+ T cells expressing eGFP after stimulation. Results are from 2 experiments, with 7 to 14 mice per group. (G) CB2R messenger RNA (mRNA) expression in B6 naïve and anti-CD3/anti-CD28 antibody activated CD4+ or CD8+ T cells. Data are presented as fold expression above 18S ribosomal RNA control and are derived from 10 to 13 mice per group. Results in panels B and D to G are presented as the mean ± standard deviation. **P < .01, ****P < .0001.
GVHD induces a loss of CB2R eGFP expression in T cells isolated from target organs. (A) Spleen sections from CB2ReGFP reporter mice were stained with fluorescence-labeled antibodies directed against eGFP, CD19, or CD3. Topro served as a nuclear stain. Merged results are shown in final panels in each row. (B-C) eGFP expression on CD4+ T cells, CD8+ T cells, and B cells derived from the spleens of normal CB2ReGFP mice (n = 16-17) are shown in panel B. Representative dot plots showing eGFP expression on these immune cell populations are depicted in panel C. (D) Lethally irradiated Balb/c mice received transplants of CB2ReGFP BM alone (n = 4) or together with CB2ReGFP spleen cells (adjusted to yield an αβ+ T-cell dose of 0.6 × 106; n = 5). Animals were euthanized 14 days posttransplantation. The eGFP expression percentage on CD4+ and CD8+ T cells obtained from spleen, liver, lung, and colon of animals receiving transplants of BM alone (BM) or together with adjunctive spleen cells (GVHD) is depicted. The eGFP expression percentage on comparable cells obtained from the spleen of normal nontransplanted CB2ReGFP mice (labeled naïve; n = 5) is shown for comparison. Statistical comparison is from all tissue sites relative to splenic CD4+ and CD8+ T cells from naïve mice. (E) Lethally irradiated Balb/c or B6.PL mice received transplants of CB2ReGFP BM and spleen cells (adjusted to yield an αβ+ T-cell dose of 0.6 × 106). The eGFP expression percentage on CD4+ and CD8+ T cells obtained from spleen, liver, and lung of animals receiving syngeneic vs allogeneic marrow grafts 14 days posttransplantation is depicted. Data are from 3 experiments, with a total of 15 mice per group. The eGFP expression percentage on T cells obtained from the spleen of normal CB2ReGFP mice is shown for comparison. (F) Magnetically purified CD4+ or CD8+ T cells (1 × 105) from CB2ReGFP mice were cultured with anti-CD3 (2.5 μg/mL) and anti-CD28 (5 μg/mL) antibody for 3 days. Magnetically purified pan T cells (1 × 105) from CB2ReGFP mice were cultured with allogeneic Balb/c CD11c–enriched dendritic cells (5 × 104) for 4 days. Data depict the percentage of CD4+ T cells and CD8+ T cells expressing eGFP after stimulation. Results are from 2 experiments, with 7 to 14 mice per group. (G) CB2R messenger RNA (mRNA) expression in B6 naïve and anti-CD3/anti-CD28 antibody activated CD4+ or CD8+ T cells. Data are presented as fold expression above 18S ribosomal RNA control and are derived from 10 to 13 mice per group. Results in panels B and D to G are presented as the mean ± standard deviation. **P < .01, ****P < .0001.
To determine whether CB2R expression was similar on immune cell populations resident in nonlymphoid tissues, we performed a similar analysis on cells isolated from the liver, lung, and colon, as well as the peripheral blood. These data revealed that B cells expressed high levels of the CB2R regardless of tissue site. However, whereas CB2R expression was low on CD4+ T cells isolated from the spleen, it was substantially higher on CD4+ T cells in the lung, liver, and peripheral blood (supplemental Figure 2). Conversely, there was also significant variability in CB2R expression on CD8+ T cells, where expression was high on splenic CD8+ T cells but significantly lower on cells isolated from the liver and colon. Thus, these data demonstrate that eGFP faithfully marks CB2R expression on immune cells and that there is substantial variability in receptor expression on adaptive immune cells in diverse tissue sites.
Interestingly, we observed that there was significant loss of eGFP expression in both CD4+ and CD8+ T cells isolated from GVHD tissue sites when compared with expression of eGFP in the same cell populations in the spleens of naïve mice (Figure 3D). This was most marked in CD8+ T cells that had higher expression of eGFP in reporter mice not undergoing transplantation (Figure 3B). Notably, similar loss of expression was observed in BM control animals that did not have clinically evident GVHD. Because it was formally possible that loss of eGFP expression in BM control mice could have been due to a lower level of alloantigen-induced proliferation as a consequence of small numbers of mature donor T cells in the marrow graft, we conducted experiments using syngeneic recipients, where donor T-cell expansion occurred only though homeostatic proliferation, to ascertain the strength of activation required to induce loss of the fluorescent signal. Interestingly, we observed that there was a significantly higher percentage of CD4+ T cells in the spleen and CD8+ eGFP+ T cells in all tissues of mice receiving syngeneic graft transplants (Figure 3E), indicating the homeostatic expansion was much less effective at inducing loss of eGFP expression. As additional confirmation, we activated T cells from CB2ReGFP animals in vitro under strong polyclonal and alloantigen-specific conditions, demonstrating a marked reduction in eGFP expression under both conditions, although loss of expression was more pronounced when T cells were stimulated with alloantigen (Figure 3F). Because there is an intervening IRES between the CB2R and eGFP genes in the targeting construct of the reporter mouse,27 it was conceivable that loss of eGFP did not signify a commensurate loss of CB2R gene expression. To address this possibility, we examined CB2R mRNA levels in CD4+ and CD8+ T cells under polyclonal activating conditions. These experiments demonstrated a significant reduction in CB2R mRNA levels in both CD4+ and CD8+ T cells (Figure 3G). We did not carry out these studies in dendritic cell coculture experiments because of the potential for confounding effects from contaminating dendritic cell mRNA. Collectively, these studies support the premise that strong T-cell activation, as occurs in GVHD, results in a substantial loss of CB2R expression on donor T cells.
Agonistic signaling through the CB2R mitigates the severity of acute GVHD
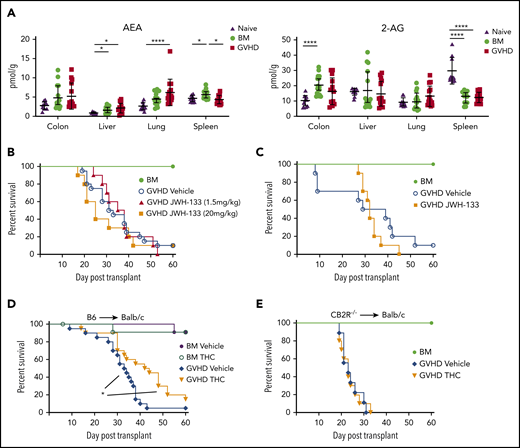
Given that loss or blockade of the CB2R worsened acute GVHD, we sought to determine whether targeted CB2R activation could mitigate GVHD severity. We first examined levels of 2-AG and AEA, which are the natural ligands for the CB2R,31 to determine if GVHD deleteriously affected endocannabinoid concentrations in target organs. We observed that endocannabinoid concentrations in tissues obtained from BM control or GVHD animals were no different or slightly higher than levels seen in normal mice, except for significantly lower 2-AG levels in the spleen (Figure 4A). Notably, quantitation of 2-AG and AEA levels revealed no difference in the concentration of either ligand in the colon, liver, or lung in GVHD when compared with BM control animals. Thus, GVHD did not result in the diminished production of these natural ligands. The effects of chronic treatment with 2 CB2R agonists, JWH-133 and THC, on GVHD severity were then examined to determine if GVHD could be ameliorated. JWH-133 is a synthetic, CB2R-selective cannabinoid that has been shown to be highly potent and efficacious in mice,28 whereas THC is a phytocannabinoid that is less selective, because it binds to both the CB1R and CB2R and has lower affinity than JWH-133.31 Cohorts of mice were treated with low (1.5 mg/kg) and high doses (20 mg/kg) of JWH-133 over the first 14 days posttransplantation, which was the same treatment schedule we employed using the CB2R antagonist SR144528 (Figure 1I). These studies demonstrated that neither JWH-133 dosing regimen resulted in improved survival (Figure 4B). Additionally, a more protracted (ie, 35 days) course of therapy also failed to protect animals from lethal GVHD (Figure 4C), as did abbreviated regimens designed to determine whether transient agonist exposure in the setting of reduced receptor expression could mitigate disease severity (supplemental Figure 3). In contrast, prolonged administration (ie, 40 days) of THC resulted in significantly improved survival relative to vehicle-treated control animals (Figure 4D). Because THC can bind to the CB1R and CB2R, it was possible that the protective effects of this agent could be mediated, in part, by binding to the CB1R. However, there was no difference in survival between THC- and vehicle-treated mice receiving CB2R−/− marrow graft transplants (Figure 4E), indicating that the beneficial effect of THC was mediated through the CB2R.
Signaling through the CB2R by THC mitigates the severity of acute GVHD. (A) Endocannabinoid levels of AEA and 2-AG in the liver, lung, colon, and spleen of lethally irradiated Balb/c mice receiving transplants of B6 BM alone or together with B6 spleen cells (adjusted to yield an αβ T-cell dose of 0.6 × 106 cells) and then undergoing assessment 14 days posttransplantation. Data are from 3 experiments, with 14 to 15 mice per group. AEA and 2-AG levels in the same tissues from naïve nontransplanted Balb/c mice are also depicted (n = 10). Data are presented as mean ± standard deviation. (B) Lethally irradiated Balb/c mice received transplants of B6 BM and spleen cells (adjusted to yield an αβ T-cell dose of 0.85 × 106 to 0.90 × 106 cells) and were then treated with a vehicle control (n = 20) or the CB2R agonist JWH-133 at a low (1.5 mg/kg; n = 10) or high dose (20 mg/kg; n = 10) for 14 consecutive days beginning on day 0. Balb/c mice receiving transplants of B6 BM alone served as controls (n = 12). Overall survival is depicted. Data are cumulative results from 4 experiments. (C) Irradiated Balb/c mice received transplants of B6 BM and spleen cells and were then treated with a vehicle control (n = 10) or the CB2R agonist JWH-133 (1.5 mg/kg; n = 10) for 35 consecutive days beginning on day 0. Balb/c mice receiving transplants of B6 BM alone served as controls (n = 6). Results are from 2 experiments. (D) Lethally irradiated Balb/c recipients received transplants of B6 BM and spleen cells (adjusted to yield an αβ T-cell dose of 0.85 × 106 to 0.9 × 106 cells). Animals were then treated with THC (20 mg/kg; n = 20) or vehicle (n = 20) for 40 days beginning on day 0. Balb/c mice receiving transplants of B6 BM alone and treatment with either vehicle or THC served as controls (n = 12 per group). Data are from 4 experiments. (E) Irradiated Balb/c mice received transplants of CB2R−/− BM alone (n = 6) or together with CB2R−/− spleen cells (adjusted to yield an αβ T-cell dose of 0.85 × 106 to 0.9 × 106 cells). Animals that received adjunctive spleen cells were treated with either THC (n = 10) or a vehicle control (n = 10) daily beginning on day 0 until death. Survival is depicted. Data are from 2 experiments. *P < .05, ****P < .0001.
Signaling through the CB2R by THC mitigates the severity of acute GVHD. (A) Endocannabinoid levels of AEA and 2-AG in the liver, lung, colon, and spleen of lethally irradiated Balb/c mice receiving transplants of B6 BM alone or together with B6 spleen cells (adjusted to yield an αβ T-cell dose of 0.6 × 106 cells) and then undergoing assessment 14 days posttransplantation. Data are from 3 experiments, with 14 to 15 mice per group. AEA and 2-AG levels in the same tissues from naïve nontransplanted Balb/c mice are also depicted (n = 10). Data are presented as mean ± standard deviation. (B) Lethally irradiated Balb/c mice received transplants of B6 BM and spleen cells (adjusted to yield an αβ T-cell dose of 0.85 × 106 to 0.90 × 106 cells) and were then treated with a vehicle control (n = 20) or the CB2R agonist JWH-133 at a low (1.5 mg/kg; n = 10) or high dose (20 mg/kg; n = 10) for 14 consecutive days beginning on day 0. Balb/c mice receiving transplants of B6 BM alone served as controls (n = 12). Overall survival is depicted. Data are cumulative results from 4 experiments. (C) Irradiated Balb/c mice received transplants of B6 BM and spleen cells and were then treated with a vehicle control (n = 10) or the CB2R agonist JWH-133 (1.5 mg/kg; n = 10) for 35 consecutive days beginning on day 0. Balb/c mice receiving transplants of B6 BM alone served as controls (n = 6). Results are from 2 experiments. (D) Lethally irradiated Balb/c recipients received transplants of B6 BM and spleen cells (adjusted to yield an αβ T-cell dose of 0.85 × 106 to 0.9 × 106 cells). Animals were then treated with THC (20 mg/kg; n = 20) or vehicle (n = 20) for 40 days beginning on day 0. Balb/c mice receiving transplants of B6 BM alone and treatment with either vehicle or THC served as controls (n = 12 per group). Data are from 4 experiments. (E) Irradiated Balb/c mice received transplants of CB2R−/− BM alone (n = 6) or together with CB2R−/− spleen cells (adjusted to yield an αβ T-cell dose of 0.85 × 106 to 0.9 × 106 cells). Animals that received adjunctive spleen cells were treated with either THC (n = 10) or a vehicle control (n = 10) daily beginning on day 0 until death. Survival is depicted. Data are from 2 experiments. *P < .05, ****P < .0001.
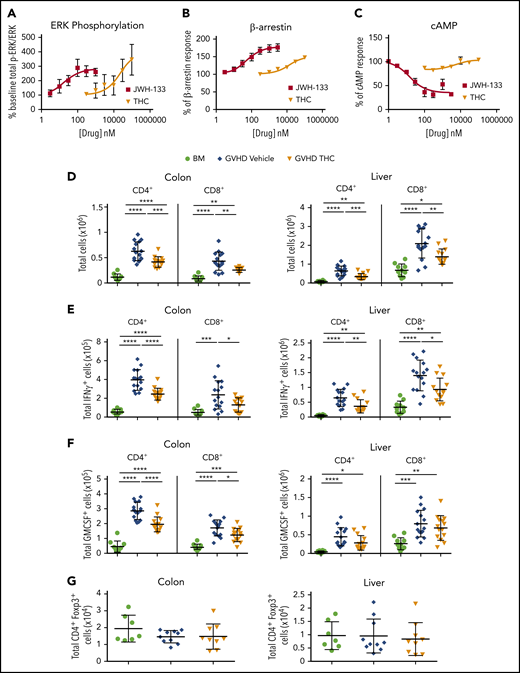
THC reduces the accumulation of proinflammatory T cells in GVHD target tissues
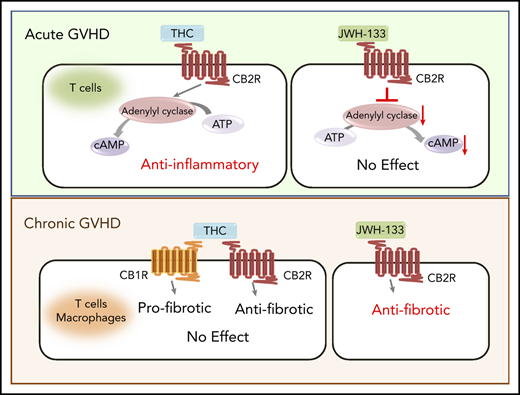
Although THC was effective at reducing GVHD severity via the CB2R signaling pathway, the highly efficacious agonist JWH-133 was without effect. To explore this paradox, we compared the signaling of these agonists using an in vitro assay. The CB2R is coupled to heterotrimeric G proteins; thus, the primary signaling pathways downstream of CB2R include inhibition of adenylyl cyclase, extracellular regulated kinase (ERK) phosphorylation, and β-arrestin recruitment.32,33 We observed that THC and JWH-133 were equally efficacious at increasing phosphorylated ERK (Figure 5A; Table 1; supplemental Figure 4). With respect to β-arrestin recruitment, both JWH-133 and THC exhibited agonist effects; however, the maximum effect (Emax) achieved by THC was significantly lower than that by JWH-133, indicating that THC is a partial agonist in this signaling pathway (Figure 5B; Table 1). Notably, JWH-133 was significantly more potent than THC in both these assays. Only JWH-133, however, exhibited agonistic activity to inhibit forskolin-induced cAMP accumulation. THC did not significantly inhibit cAMP accumulation at concentrations up to 30 µM, and even exhibited a slight increase in cAMP accumulation (Figure 5C; Table 1). These findings suggest that the ability of THC to reduce GVHD lethality is due to a signaling bias that results in a lack of effect on cAMP homeostasis while other CB2R signaling pathways are engaged.
THC reduces proinflammatory T-cell accumulation in GVHD target organs. (A-C) PathHunter Chinese hamster ovary (CHO)-K1 CNR2 cells stably expressing the human CB2 receptor were used for signaling assays. Ratio of phosphorylated ERK (p-ERK) to total ERK (A), percentage of β-arrestin recruitment (B), and inhibition percentage of forskolin-induced cyclic adenosine monophosphate (cAMP) production (C) as a function of varying concentrations of JWH-133 and THC are depicted. The 50% effective concentration/50% inhibitory concentration and maximum effect values calculated from these curves are reported in Table 1. Symbols in panels A to C represent the mean from 3 to 6 determinations per assay. Lines drawn are the least squares best fit of the data to the nonlinear, 3-parameter log concentration-response relationship, where bottom (for p-ERK/ERK and β-arrestin) or top (for cAMP) values were constrained to 100. (D-G) Lethally irradiated Balb/c recipients received transplants of B6 BM and spleen cells (adjusted to yield an αβ T-cell dose of 0.85 × 106 to 0.9 × 106 cells). Animals were treated with THC (20 mg/kg; n = 9-14) or vehicle control (n = 10-15) for 21 days beginning on day 0. Balb/c mice receiving transplants of B6 BM alone served as controls (n = 7-10). The total numbers of CD4+ and CD8+ T cells in the colon and liver 21 days posttransplantation are shown in panel D. The absolute numbers of CD4+ or CD8+ T cells that produced IFN-γ (E) or GM-CSF (F) are depicted in these tissue sites. Total numbers of CD4+ Foxp3+ T cells in the colon or liver (G). Data are presented as the mean ± standard deviation and are cumulative results from 2 to 3 experiments. *P < .05, **P < .01, ***P < .001, ****P < .0001.
THC reduces proinflammatory T-cell accumulation in GVHD target organs. (A-C) PathHunter Chinese hamster ovary (CHO)-K1 CNR2 cells stably expressing the human CB2 receptor were used for signaling assays. Ratio of phosphorylated ERK (p-ERK) to total ERK (A), percentage of β-arrestin recruitment (B), and inhibition percentage of forskolin-induced cyclic adenosine monophosphate (cAMP) production (C) as a function of varying concentrations of JWH-133 and THC are depicted. The 50% effective concentration/50% inhibitory concentration and maximum effect values calculated from these curves are reported in Table 1. Symbols in panels A to C represent the mean from 3 to 6 determinations per assay. Lines drawn are the least squares best fit of the data to the nonlinear, 3-parameter log concentration-response relationship, where bottom (for p-ERK/ERK and β-arrestin) or top (for cAMP) values were constrained to 100. (D-G) Lethally irradiated Balb/c recipients received transplants of B6 BM and spleen cells (adjusted to yield an αβ T-cell dose of 0.85 × 106 to 0.9 × 106 cells). Animals were treated with THC (20 mg/kg; n = 9-14) or vehicle control (n = 10-15) for 21 days beginning on day 0. Balb/c mice receiving transplants of B6 BM alone served as controls (n = 7-10). The total numbers of CD4+ and CD8+ T cells in the colon and liver 21 days posttransplantation are shown in panel D. The absolute numbers of CD4+ or CD8+ T cells that produced IFN-γ (E) or GM-CSF (F) are depicted in these tissue sites. Total numbers of CD4+ Foxp3+ T cells in the colon or liver (G). Data are presented as the mean ± standard deviation and are cumulative results from 2 to 3 experiments. *P < .05, **P < .01, ***P < .001, ****P < .0001.
Potency and efficacy of JWH-133 and THC in CB2R signaling response assays
| . | ERK phosphorylation . | β-arrestin2 recruitment . | Inhibition of cAMP . | |||
|---|---|---|---|---|---|---|
| EC50**** . | Emax, % . | EC50**** . | Emax, %** . | IC50 . | Maximum inhibition . | |
| JWH-133 | 19 ± 2 nM | 340 ± 63 | 49 ± 1 nM | 177 ± 6 | 16 ± 1 nM | 35 ± 4 |
| THC | 23 ± 3 µM | 475 ± 158 | 13 ± 1 µM | 144 ± 3 | Inactive | Inactive |
| . | ERK phosphorylation . | β-arrestin2 recruitment . | Inhibition of cAMP . | |||
|---|---|---|---|---|---|---|
| EC50**** . | Emax, % . | EC50**** . | Emax, %** . | IC50 . | Maximum inhibition . | |
| JWH-133 | 19 ± 2 nM | 340 ± 63 | 49 ± 1 nM | 177 ± 6 | 16 ± 1 nM | 35 ± 4 |
| THC | 23 ± 3 µM | 475 ± 158 | 13 ± 1 µM | 144 ± 3 | Inactive | Inactive |
CHO cells were stably transfected with the human CB2R and then used in PathHunter assays to assess the effect of varying concentrations of JWH-133 and THC on specified signaling pathways. Data are from 3 to 6 replicates per assay and are presented as the mean ± standard error of the mean.
EC50, 50% effective concentration; IC50, 50% inhibitory concentration.
P < .01, ****P < .0001.
Because cAMP is a mechanism by which regulatory T cells (Tregs) mediate suppressive effects34 and is able to inhibit effector T-cell responses,35 we examined how THC ameliorated GVHD by quantifying the number of proinflammatory T cells and Tregs in the primary GVHD target tissues affected by CB2R signaling (ie, colon and liver; Figure 2). Mice treated with THC had a significant reduction in the absolute number of donor CD4+ and CD8+ T cells in the colon and liver (Figure 5D). In addition, there was a statistically significant reduction in the number of CD4+ and CD8+ T cells that produced IFN-γ in the liver and colon (Figure 5E) and in the number of CD4+ and CD8+ T cells that made GM-CSF in the gastrointestinal tract (Figure 5F). Notably, there was no effect on Treg numbers (Figure 5G), indicating that THC reduced proinflammatory T-cell accumulation in target organs but did not augment Treg reconstitution.
Signaling through the CB2R regulates sclerodermatous chronic skin GVHD
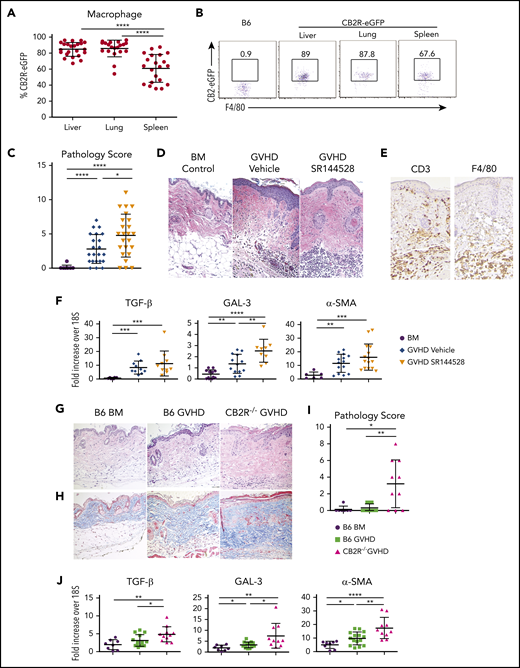
Whereas acute GVHD is driven primarily by alloreactive donor T cells, chronic GVHD is characterized by additional critical contributions from the innate immune system. Macrophages, in particular, have a pivotal role in the development of fibrosis, which is a cardinal feature of this disease.4,8,36 We observed that the CB2R was also expressed on macrophages from naïve CB2ReGFP animals, albeit with some variability in different tissue sites (Figure 6A-B). To determine the role of CB2R signaling in chronic GVHD, we used 2 murine sclerodermatous chronic GVHD models.4,37,38 We first used a pharmacological approach, which demonstrated that mice administered the CB2R antagonist SR144528 had a significant increase in overall pathological score (Figure 6C), as evidenced by hyperkeratosis in the epidermis along with increased inflammatory cell infiltrates in the dermis (Figure 6D). Notably, immunohistochemical staining revealed that dermal infiltration was attributable to the accumulation of both CD3+ T cells and F4/80+ macrophages in antagonist-treated mice (Figure 6E), indicating that there were contributions from both cell populations in pathologically involved tissue. Given the presence of macrophages in the skin, we isolated these cells from more easily accessible sites (ie, lung and peritoneal cavity) to determine whether administration of a CB2R antagonist altered macrophage phenotype or function. We observed that there was no difference in expression of MHC class II or costimulatory molecules (supplemental Figure 5A) or production of interleukin-1β or GM-CSF (supplemental Figure 5B) in macrophages obtained from antagonist- vs vehicle control–treated mice. We then obtained skin samples from these mice and examined genes that are expressed by macrophages and have been shown to be important in the pathophysiology of fibrosis in nontransplantation models.39-41 These studies demonstrated that GVHD control mice had a significant increase in mRNA levels of TGF-β, GAL-3, and α-SMA compared with BM animals (Figure 6F). Notably, the administration of a CB2R antagonist resulted in a further increase in the expression of GAL-3 when compared with vehicle-treated mice, indicating that inhibition of CB2R signaling promoted skin expression of a gene implicated in the development of fibrosis.
Signaling through the CB2R regulates the severity of chronic GVHD. (A-B) The eGFP expression percentage on macrophages derived from the liver, lung, or spleen of normal CB2ReGFP mice (n = 20-23) is shown. Representative dot plots showing eGFP expression on macrophages from these tissue sites is shown in panel B. (C-D) Lethally irradiated Balb/c mice received transplants of B10.D2 BM alone (n = 9) or together with 15 × 106 B10.D2 spleen cells. Animals that received spleen cells were treated with the CB2R antagonist SR144528 (3 mg/kg; n = 24) or a vehicle control (n = 22) for 14 days beginning on the day of transplantation. Cumulative pathological chronic GVHD skin scores of mice 30 to 40 days posttransplantation are depicted in panel C. Representative hematoxylin and eosin–stained sections of the skin on day 35 are shown in panel D. Original magnification is ×100 for photomicrographs. Data are derived from 5 experiments. (E) Immunohistochemical staining depicting CD3 and F4/80 positive cells (brown coloration) in the skin of mice treated with SR144528. (F) mRNA expression of transforming growth factor β (TGF-β), galectin 3 (GAL-3), and α smooth muscle (α-SMA) in the skin of animals 30 to 40 days posttransplantation. Results are from 3 experiments, with 6 to 15 mice per group. (G-J) Lethally irradiated (850 cGy) Balb/c mice received transplants of B6 Rag-1−/− BM alone, B6 Rag-1−/− BM and B6 spleen cells (adjusted to yield an αβ T-cell dose of 0.4 × 106), or CB2R−/− BM and spleen cells (adjusted to yield the same αβ T-cell dose). Representative hematoxylin and eosin– and trichrome-stained sections of the skin on day 45 are shown in panels G and H, respectively. Original magnification is ×100 for photomicrographs. (I) Pathology scores of the skin 40 to 45 days posttransplantation in chronic GVHD mice. Results are from 3 experiments, with 7 to 13 mice per group. (J) mRNA expression of TGF-β, GAL-3, and α-SMA in the skin of animals (n = 8-15 mice per group) 40 to 45 days posttransplantation. Results are from 3 experiments. Data in panels in A, C, F, I, and J are presented as the mean ± standard deviation. *P < .05, **P < .01, ***P < .001, ****P < .0001.
Signaling through the CB2R regulates the severity of chronic GVHD. (A-B) The eGFP expression percentage on macrophages derived from the liver, lung, or spleen of normal CB2ReGFP mice (n = 20-23) is shown. Representative dot plots showing eGFP expression on macrophages from these tissue sites is shown in panel B. (C-D) Lethally irradiated Balb/c mice received transplants of B10.D2 BM alone (n = 9) or together with 15 × 106 B10.D2 spleen cells. Animals that received spleen cells were treated with the CB2R antagonist SR144528 (3 mg/kg; n = 24) or a vehicle control (n = 22) for 14 days beginning on the day of transplantation. Cumulative pathological chronic GVHD skin scores of mice 30 to 40 days posttransplantation are depicted in panel C. Representative hematoxylin and eosin–stained sections of the skin on day 35 are shown in panel D. Original magnification is ×100 for photomicrographs. Data are derived from 5 experiments. (E) Immunohistochemical staining depicting CD3 and F4/80 positive cells (brown coloration) in the skin of mice treated with SR144528. (F) mRNA expression of transforming growth factor β (TGF-β), galectin 3 (GAL-3), and α smooth muscle (α-SMA) in the skin of animals 30 to 40 days posttransplantation. Results are from 3 experiments, with 6 to 15 mice per group. (G-J) Lethally irradiated (850 cGy) Balb/c mice received transplants of B6 Rag-1−/− BM alone, B6 Rag-1−/− BM and B6 spleen cells (adjusted to yield an αβ T-cell dose of 0.4 × 106), or CB2R−/− BM and spleen cells (adjusted to yield the same αβ T-cell dose). Representative hematoxylin and eosin– and trichrome-stained sections of the skin on day 45 are shown in panels G and H, respectively. Original magnification is ×100 for photomicrographs. (I) Pathology scores of the skin 40 to 45 days posttransplantation in chronic GVHD mice. Results are from 3 experiments, with 7 to 13 mice per group. (J) mRNA expression of TGF-β, GAL-3, and α-SMA in the skin of animals (n = 8-15 mice per group) 40 to 45 days posttransplantation. Results are from 3 experiments. Data in panels in A, C, F, I, and J are presented as the mean ± standard deviation. *P < .05, **P < .01, ***P < .001, ****P < .0001.
We then used a complementary genetic approach in which Balb/c mice received Rag-1−/− BM transplants and a low dose of T cells from either WT B6 or CB2R−/− animals.38 We observed increased pathological damage in the skin of recipient mice receiving CB2R−/− marrow grafts, as evidenced by dermal fibrosis, inflammatory infiltrates, and loss of hair follicles (Figure 6G). Trichrome staining also demonstrated augmented collagen deposition in these animals (Figure 6H). Overall, there was a significant increase in pathological damage (Figure 6I) when compared with GVHD controls. In addition, animals reconstituted with CB2R−/− marrow grafts had augmented mRNA levels of TGF-β, GAL-3, and α-SMA relative to mice receiving WT grafts (Figure 6J). Collectively, these results demonstrated that pharmacological and genetic inhibition of CB2R signaling exacerbated sclerodermatous chronic GVHD.
Agonistic signaling through the CB2R mitigates the severity of chronic GVHD
Based on these results, we examined whether administration of a CB2R agonist could mitigate chronic GVHD in a model characterized by pathophysiological contributions from both T cells and macrophages (Figure 6E). Interestingly, we observed that there was no loss of eGFP expression on macrophages isolated from the spleen, lung, or liver of GVHD animals compared with nontransplantation or BM control CB2ReGFP mice (Figure 7A-B). Consistent with that finding in reporter mice, mRNA levels of CB2R in flow-sorted macrophages obtained from the spleen were no different between BM controls and GVHD WT mice (Figure 7C). Thus, CB2R expression seemed more stable on macrophages than on T cells under inflammatory conditions. Given this disparity, we examined the relative efficacy of THC vs JWH-133 in mitigating disease severity. In contrast to what we observed during acute GVHD, JWH-133 administration resulted in a significant decrease in pathological damage, whereas THC had no protective effect in chronic GVHD (Figure 7D-E). In addition, there was a commensurate reduction in gene expression of TGF-β and α-SMA in the skin of JWH-133–treated mice, whereas THC administration had no effect on gene expression relative to GVHD control animals (Figure 7F). Thus, these studies demonstrated that JWH-133 was more efficacious than THC in a chronic GVHD model and that these agonists had differential effects on acute and chronic GVHD.
JWH-133, but not THC, mitigates the severity of chronic GVHD. (A-C) Lethally irradiated Balb/c mice received transplants of CB2ReGFP BM alone or together with spleen cells (adjusted to yield an αβ T-cell dose of 0.6 × 106). Macrophages were isolated from the liver, lung, and spleen of mice 14 days posttransplantation. eGFP expression on macrophages from BM control and GVHD mice is shown in panel A. Naïve CB2ReGFP mice not undergoing transplantation are shown for comparison. Results are from 4 to 5 mice per group. Representative histogram of CB2R-eGFP expression on splenic macrophages from mice in panel B. Normal B6 mice and naïve CB2ReGFP mice not undergoing transplantation are shown for comparison. CB2R mRNA expression from flow-sorted CD11b+ F4/80+ macrophages obtained from the spleen on day 14 is depicted in panel C. Results for GVHD mice were pooled from 2 to 3 mice per data point. (D-F) Lethally irradiated Balb/c mice received transplants of B10.D2 BM alone or with 20 × 106 spleen cells. Animals that received spleen cells were treated with JWH-133 (1.5 mg/kg), THC (20 mg/kg), or a vehicle control for 35 to 40 days beginning on day 0. Representative hematoxylin and eosin–stained sections of the skin on day 35 are shown in panel D, and cumulative pathological skin scores from replicate animals 35 to 40 days posttransplantation are depicted in panel E. Original magnification is ×100 for photomicrographs. (F) mRNA expression of TGF-β, GAL-3, and α-SMA in the skin of animals (n = 7-15 mice per group) 35 to 40 days posttransplantation. Results are from 6 experiments. Data in panels A, C, E, and F are presented as the mean ± standard deviation. *P < .05, ** P < .01, ***P < .001.
JWH-133, but not THC, mitigates the severity of chronic GVHD. (A-C) Lethally irradiated Balb/c mice received transplants of CB2ReGFP BM alone or together with spleen cells (adjusted to yield an αβ T-cell dose of 0.6 × 106). Macrophages were isolated from the liver, lung, and spleen of mice 14 days posttransplantation. eGFP expression on macrophages from BM control and GVHD mice is shown in panel A. Naïve CB2ReGFP mice not undergoing transplantation are shown for comparison. Results are from 4 to 5 mice per group. Representative histogram of CB2R-eGFP expression on splenic macrophages from mice in panel B. Normal B6 mice and naïve CB2ReGFP mice not undergoing transplantation are shown for comparison. CB2R mRNA expression from flow-sorted CD11b+ F4/80+ macrophages obtained from the spleen on day 14 is depicted in panel C. Results for GVHD mice were pooled from 2 to 3 mice per data point. (D-F) Lethally irradiated Balb/c mice received transplants of B10.D2 BM alone or with 20 × 106 spleen cells. Animals that received spleen cells were treated with JWH-133 (1.5 mg/kg), THC (20 mg/kg), or a vehicle control for 35 to 40 days beginning on day 0. Representative hematoxylin and eosin–stained sections of the skin on day 35 are shown in panel D, and cumulative pathological skin scores from replicate animals 35 to 40 days posttransplantation are depicted in panel E. Original magnification is ×100 for photomicrographs. (F) mRNA expression of TGF-β, GAL-3, and α-SMA in the skin of animals (n = 7-15 mice per group) 35 to 40 days posttransplantation. Results are from 6 experiments. Data in panels A, C, E, and F are presented as the mean ± standard deviation. *P < .05, ** P < .01, ***P < .001.
Discussion
The complex interplay between cells of the adaptive and innate arms of the immune system is a critical aspect of GVHD pathophysiology. Although T cells have long been accepted as primary drivers of GVHD biology, innate immune cells are increasingly being recognized as having important roles in acute and, particularly, chronic GVHD.4,5,8,9 Therefore, therapeutic strategies that target immune cells resident in both compartments have the potential to have a salutary impact on this disease. In the current study, we examined the functional role of the CB2R, which is expressed on both T cells and macrophages, in complementary murine models where both cell types contributed to disease pathogenesis. These results demonstrated that the CB2R plays an important role in the regulation of GVHD and that the administration of CB2R agonists can be used as a therapeutic strategy to mitigate disease severity.
The fact that the CB2R was differentially expressed on CD8+ vs CD4+ T cells (ie, ∼90% vs ∼20%) suggested that the receptor might be disproportionately important in the regulation of CD8+ T-cell alloreactivity. This was apparent from acute GVHD studies in which transplantation with CB2R−/− marrow grafts resulted only in an increased accumulation of CD8+ T cells with a proinflammatory phenotype, with no effect on CD4+ T cells. Surprisingly, when we examined the specific contribution of CB2R expression on CD4+ vs CD8+ T cells, we observed that absence of the receptor on either population was sufficient to exacerbate GVHD lethality in the presence of the corresponding WT population. However, this was not attributable to any discernible alteration in the proinflammatory profile of CD4+ T cells, because neither transplantation with CD4+ CB2R−/− nor transplantation with CD8+ CB2R−/− T cells promoted CD4+ T-cell expansion or inflammatory cytokine production. Rather, absence of CB2R expression on either T-cell population only promoted the accumulation and production of inflammatory cytokines by CD8+ T cells within GVHD target tissues. Interestingly, histological examination did reveal that absence of the CB2R on CD4+ T cells resulted in worse pathological damage in the liver. The increased expression of the receptor on CD4+ T cells in the liver, relative to other tissue sites, is a potential explanation for this finding and may be indicative of an important role for the CB2R in regulating immune responses within this tissue. Overall, however, the predominant observation from these studies was that CB2R expression, particularly on CD8+ T cells, was critical for the regulation of CD8+ T-cell alloreactivity during acute GVHD.
Our data demonstrated substantial loss of eGFP expression and a significant reduction in CB2R mRNA levels during acute GVHD, both of which were consistent with a loss of CB2R expression on the cell surface of T cells. However, our finding that treatment with an antagonist or the complete genetic loss of donor CB2R exacerbated GVHD suggests that sufficient CB2Rs remain on T cells that could potentially be activated to reduce disease by an appropriate agonist. To test this hypothesis, we examined the ability of 2 CB2R agonists, JWH-133 and THC, to ameliorate GVHD severity. JWH-133, a synthetic cannabinoid, was used because it is reported to be a selective CB2R full agonist with minimal off-target effects and relatively balanced activation of signal transduction pathways, except for little activity in the recruitment of β-arrestin, suggesting it might not further downregulate CB2R function.28 THC, a phytocannabinoid with much lower affinity for the CB2R than JWH-133 but efficacy in at least some signaling assays,28 was shown to reduce weight loss and splenomegaly in a nonirradiated GVHD model.42 Surprisingly, we observed that JWH-133 failed to protect animals from lethal acute GVHD, despite using multiple dosing and administration schedules. Conversely, GVHD mice treated with THC exhibited a significant increase in survival when compared with vehicle-treated GVHD control animals. Because THC can bind to both the CB1R and CB2R, we considered that binding to the CB1R could have played a role in mitigating inflammation. However, the administration of THC to mice reconstituted with CB2R−/− grafts failed to prolong survival, indicating that THC exerted immune suppressive effects through the CB2R.
Biased agonism is a characteristic of CB2R agonists whereby the primary intracellular signaling pathways (β-arrestin signaling, inhibition of adenylyl cyclase, and activation of ERK) are selectively engaged, which can lead to differential biological effects.32 To understand why JWH-133 and THC administration had divergent results with respect to protection from acute GVHD, we performed in vitro assays using an engineered cell line expressing human CB2R to compare the effects of JWH-133 and THC on these major signaling pathways. Our data demonstrated that both JWH-133 and THC effectively activated CB2R signaling cascades that resulted in β-arrestin recruitment and activation of ERK. We observed that JWH-133 was considerably more potent than THC and that THC exhibited the characteristic of a partial agonist in the β-arrestin recruitment assay. Notably, whereas JWH-133 inhibited adenylyl cyclase, THC had no measurable effect on this pathway, which is in accordance with earlier studies.43 Our data showing that THC was a full agonist in some CB2R-mediated signaling assays but had very low efficacy in others suggest that it is a protean ligand. Protean ligands are receptor agonists that can activate signaling cascades that are inactive when the receptor is unbound; however, the efficacy of these ligands is too low to affect signaling cascades that are constitutively active.44 Mechanistically, from a GVHD perspective, adenylyl cyclase is responsible for the synthesis of cAMP, which is critical for maintaining immune regulation35 and is a primary pathway by which regulatory T cells mediate their suppressive effects.34 Furthermore, cAMP is also capable of inhibiting effector T cell–mediated inflammatory responses.35 One potential mechanism for the superiority of THC over JWH-133 is that although both agonists activate CB2R-mediated signaling in T cells, resulting in anti-inflammatory effects, JWH-133 also reduces cAMP-mediated signaling, which exerts proinflammatory effects in opposition to the positive effects of CB2R activation. Because THC does not inhibit adenylyl cyclase, it can activate the beneficial signaling cascade without the opposing effect to reduce cAMP concentrations. An alternative explanation is that the 2 ligands result is different rates of removal and replacement of the CB2R on the cell surface. These and other potential mechanistic insights await further study.
Numerous studies in nontransplantation murine models of liver and dermal fibrosis have demonstrated that endocannabinoids that signal through the CB2R can exert antifibrotic effects.45-47 Consistent with these data, we observed that the genetic absence of the CB2R on donor T cells as well as CB2R antagonism exacerbated disease severity in 2 sclerodermatous chronic GVHD models. Notably, however, the administration of THC, which prolonged survival in acute GVHD, had no salutary effect in chronic GVHD. Conversely, JWH-133 resulted in significantly reduced inflammation, as determined by pathological assessment and quantification of genes associated with the development of fibrosis, despite its lack of efficacy in acute GVHD. There are several possible explanations for these divergent results. One is that whereas acute GVHD is primarily a T cell–dependent process, the pathophysiology of chronic GVHD is characterized by prominent contributions from cells of the innate immune system, particularly macrophages. To that end, our studies revealed that macrophages, unlike T cells, retained CB2R expression when directly isolated from GVHD mice. It is therefore possible that the signaling cascade elicited by JWH-133 is more effective than THC in macrophages. An alternative, perhaps more likely, explanation, however, is that THC binds to both the CB1R and CB2R. Whereas ligation of the CB2R is antifibrogenic, binding of agonists to the CB1R promotes fibrogenesis,48,49 in part by activation of IRF5.50,51 Thus, nonselectivity of THC for the CB2R could explain its lack of observed efficacy in comparison with JWH-133 in chronic GVHD.
In summary, these studies identify the CB2R as a G protein–coupled receptor expressed on cells of the adaptive and innate arms of the immune system that can regulate the severity of acute and chronic GVHD. Effective therapeutic targeting of the CB2R was dependent upon agonist signaling characteristics and receptor selectivity in conjunction with the specific composition of pathogenic immune effector cells in involved tissues. The growing interest in the development of selective and specialized ligands that target the CB2R raises the possibility that pharmacological agonists will be available to mitigate GVHD-associated inflammation in human allogeneic HSCT patients. In addition, the availability of dronabinol as an agent to treat chemotherapy-induced nausea and stimulate appetite may provide the opportunity for this drug to be repurposed to determine whether THC can prevent acute GVHD in carefully selected patient populations undergoing HSCT.
For original data, please e-mail the corresponding author.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This research was supported by grants from the Ministerio de Economía y Competitividad (SAF 2016/75959) and Ministerio de Educación of Spain (PR2009-0169) (J.R.), National Institutes of Health, National Heart, Lung, and Blood Institute grants R01 HL139008 (W.R.D. and C.J.H.) and R01 HL126166 (W.R.D.), and the Research Component of the Advancing a Healthier Wisconsin Endowment at the Medical College of Wisconsin (C.J.H.).
Authorship
Contribution: C.Y.Y. conducted and designed experiments and wrote the manuscript; V.Z. and T.S. performed experiments; G.S. conducted mass spectrometry studies; R.K. performed blinded pathological analysis; A.L., R.M.T., and J.R. conducted immunofluorescence analyses; C.J.H. designed experiments, supervised studies, and edited the paper; and W.R.D. designed experiments, supervised the study, and wrote the manuscript.
Conflict-of-interest disclosure: C.J.H. has a stockholder interest in Formulate Biosciences. The remaining authors declare no competing financial interests.
Correspondence: William R. Drobyski, Bone Marrow Transplant Program, 8701 Watertown Plank Rd, Milwaukee, WI 53226; e-mail: wdrobysk@mcw.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal