Key Points
Rate of reduction in BCR-ABL1 transcripts over the first 3 months of therapy is a strong predictor of sustained TFR.
Slower rates of BCR-ABL1 decline correlate with longer duration of drug exposure to become eligible for a TFR attempt.
Abstract
With treatment-free remission (TFR) rapidly becoming the ultimate goal of therapy in chronic myeloid leukemia (CML), there is a need to develop strategies to maximize sustained TFR by improving our understanding of its key determinants. Chronic-phase CML patients attempting TFR were evaluated to identify the impact of multiple variables on the probability of sustained TFR. Early molecular response dynamics were included as a predictive variable, assessed by calculating the patient-specific halving time of BCR-ABL1 after commencing tyrosine kinase inhibitor (TKI) therapy. Overall, 115 patients attempted TFR and had ≥12 months of follow-up. The probability of sustained TFR, defined as remaining in major molecular response off TKI therapy for 12 months, was 55%. The time taken for the BCR-ABL1 value to halve was the strongest independent predictor of sustained TFR: 80% in patients with a halving time of <9.35 days (first quartile) compared with only 4% if the halving time was >21.85 days (last quartile) (P < .001). The e14a2 BCR-ABL1 transcript type and duration of TKI exposure before attempting TFR were also independent predictors of sustained TFR. However, the BCR-ABL1 value measured at 3 months of TKI was not an independent predictor of sustained TFR. A more rapid initial BCR-ABL1 decline after commencing TKI also correlated with an increased likelihood of achieving TFR eligibility. The association between sustained TFR and the time taken for BCR-ABL1 to halve after commencing TKI was validated using an independent dataset. These data support the critical importance of the initial kinetics of BCR-ABL1 decline for long-term outcomes.
Introduction
Sustained treatment-free remission (TFR), defined as remaining off tyrosine kinase inhibitor (TKI) therapy in a major molecular response (MMR; BCR-ABL1 ≤0.1% on the international scale [IS]), is increasingly accepted as the ultimate therapeutic goal for patients diagnosed with chronic-phase chronic myeloid leukemia (CP-CML) in routine clinical practice.1-3 To date, the published success rate of TFR attempts, that is TKI discontinuation in a stable deep molecular response (DMR; defined as either MR4.0, BCR-ABL1IS ≤0.01% or MR4.5, BCR-ABL1IS ≤0.0032%) leads to sustained TFR in 40% to 65% of patients.4-8 Understanding TFR biology may assist in determining why particular patients remain in a sustained TFR, whereas others experience molecular relapse. TKI discontinuation trials4,5,7,8 have investigated numerous parameters of patients attempting TFR, with the aim of identifying the key predictors of successful drug discontinuation to improve patient selection and maximize the chances of sustained TFR.
Currently, few clinical variables demonstrate a consistent relationship with sustained TFR. The strongest to date are durations of TKI therapy and DMR before a TFR attempt. The French Stop Imatinib (STIM) study7 demonstrated a reduced risk of molecular relapse with imatinib exposure of >54 months. The interim analysis of the European Stop Kinase Inhibitor (Euro-SKI) study4 validated the association and demonstrated that patients with >5.8 years of TKI exposure had a lower risk of molecular relapse. The Korean Imatinib Discontinuation (KID)9 and De-Escalation and Stopping Treatment with Imatinib, Nilotinib, or sprYcel (DESTINY)10 studies confirmed the link between TKI duration and sustained TFR. Likewise, a longer duration of nilotinib treatment before the TFR attempt, rather than total TKI exposure, was associated with a higher rate of sustained TFR in the Evaluating Nilotinib Efficacy and Safety in Clinical Trials: Freedom (ENESTfreedom) trial.11 However, duration of DMR was a stronger predictor of TFR outcomes in the Euro-SKI study where >3.1 years of MR4.0 was associated with a reduced molecular relapse rate at 6 months.4 Similarly, duration of MR4.5 prior to the TFR attempt predicted molecular relapse in the ENESTfreedom trial.11
The majority of TFR studies have enrolled patients treated with the first-generation TKI, imatinib. First-line treatment with second-generation TKIs (2G-TKIs) lead to more rapid DMR,12,13 which could increase the rate of TFR eligibility over the first 5 years of therapy.14 However, the overall rate of sustained TFR is similar.8,15 Older age was associated with increased sustained TFR in some clinical trials,16,17 but was not validated in other studies. Withdrawal syndrome secondary to TKI cessation had a weak association with sustained TFR in the KID study,9 but has not been reported consistently. A weak association between high Sokal risk and molecular relapse was demonstrated in the Australian CML8 TWISTER study,5 supporting similar findings from the STIM trial.7 The BCR-ABL1 e14a2 transcript has been associated with higher rates of sustained TFR in small clinical series.18,19 Other biological variables were identified as predictors of TFR outcomes in individual studies but require further validation before being translated into clinical practice.20-25
Understanding the variables that predict TFR are critical for treatment decisions. The proportion of patients who achieve a sustained TFR depends on 2 key factors: the proportion of patients who achieve TFR eligibility and the proportion of TFR-eligible patients who discontinue TKI and remain in MMR. An early molecular response (EMR, BCR-ABL1IS <10% at 3 months of first-line TKI therapy) predicts subsequent DMR.26 The initial rate of BCR-ABL1 decline also influences outcomes. The German CML-IV study demonstrated that the relative change in BCR-ABL1 value at 3 months of TKI therapy from the baseline value was associated with superior progression-free survival.27 However, fold-reduction and absolute BCR-ABL1 values measured at a defined time point fail to account for the influence of time, which can impact outcomes.28 A 3-month BCR-ABL1 value could theoretically be measured between 1.5 and 4.5 months,29 whereas BCR-ABL1 halving time measurements (supplemental Figure 1 on the Blood Web site) account for variability in the timing of the 3-month sample collection and consistently predict MMR and DMR in multiple studies.28,30-35 We explored the relationship between early response kinetics and sustained TFR.
Patients and methods
We performed a retrospective analysis of all adult patients receiving their primary CML management at 2 university hospitals in South Australia between January 2008 and October 2019, including patients diagnosed before 2008 who continued to be managed at these institutions. Ethics approval was obtained from the local institutional review board and the study was performed in accordance with the Declaration of Helsinki. Clinical and laboratory records were reviewed and collated. Twenty patients were enrolled in the Australasian Leukaemia and Lymphoma Group CML8 TWISTER study (ACTRN 12606000118505). The remaining patients discontinued TKI outside the setting of a clinical trial based on the physician’s recommendation and patient preference. The criteria for TFR eligibility (supplemental Methods) were adapted from the CML8 study,5 and included a minimum of 3 years of total TKI therapy and 2 years of sustained MR4.5. MR4.5 was confirmed by a minimum of 4 tests over 2 years. Nonquantifiable transcripts or a history of either blast crisis or accelerated phase CML were exclusion criteria for a TFR attempt. Validation of identified predictors of TFR outcome was performed on an independent dataset provided by The Catholic University of Korea, South Korea.
Molecular monitoring
Patients attempting TFR in South Australia were monitored with BCR-ABL1 quantitative reverse transcription-polymerase chain reaction (PCR) in a single laboratory.36 The ratio of BCR-ABL1 to the control gene, BCR, was converted to the BCR-ABL1IS using the laboratory-specific conversion factor.37 The consistency and reliability of this assay has been demonstrated, confirming its suitability for assessment of BCR-ABL1 molecular dynamics.26,28,37-40 Patients included in the validation cohort were monitored with an ABL1-based assay.41 All patients attempting TFR underwent monthly BCR-ABL1 testing for a minimum of 12 months. Sustained TFR was defined as remaining off TKI with preservation of MMR at 12 months. TKI treatment was reinitiated for loss of MMR (molecular relapse) on a single test.6 The TKI restart criteria for the 20 patients enrolled in the CML8 study5 was more stringent and required TKI recommencement for positive BCR-ABL1 values on 2 consecutive tests or loss of MMR. Patients recommencing TKI with BCR-ABL1 values <0.1% were treated the same as patients recommencing TKI for MMR loss.
BCR-ABL1 rate of decline measurements
Halving time was calculated from available baseline (sample measured at or soon after diagnosis and before commencement of any TKI therapy) and 3-month BCR-ABL1 values using the formula here. Further detail is provided in supplemental Methods and supplemental File 2.28 Shorter halving times indicate more rapid BCR-ABL1 decline.
where x is the number of days between the baseline and 3-month assessment; y is the baseline BCR-ABL1 value; and z is the 3-month BCR-ABL1 value.
The BCR-ABL1 fold-reduction was derived from the baseline BCR-ABL1 value divided by the 3-month value. Unlike halving time, this calculation does not incorporate the time difference between the 2 measurements, which varied by almost 60 days (Table 1). This variability can have a major impact on the calculated rate of BCR-ABL1 decline.28
Characteristics of all CML patients attempting TFR with ≥12 months of follow-up (n = 115)
| Patient characteristic . | Patients attempting TFR with ≥12-mo follow-up No. (%) or median [range] . |
|---|---|
| Male | 61 (53.0) |
| Age at TFR attempt, y | 60.9 [33.4-89.8] |
| Sokal score | |
| Low-risk (<0.8) | 49 (42.6) |
| Intermediate-risk (0.8-1.2) | 41 (35.7) |
| High-risk (>1.2) | 17 (14.8) |
| Not available | 8 (7.0) |
| ELTS score | |
| Low-risk (≤1.5680) | 72 (63.2) |
| Intermediate-risk (>1.5680-≤2.2185) | 26 (22.4) |
| High-risk (>2.2185) | 8 (6.4) |
| Not available | 9 (8.0) |
| Transcript type | |
| e14a2 | 51 (44.3) |
| e13a2 | 43 (37.4) |
| Both e14a2 and e13a2 | 20 (17.4) |
| e1a2 | 1 (0.9) |
| Time to MR4.5, y | 2.2 [0.2-13.2] |
| Duration of MR4.5, y | 3.3 [2.0-12.5] |
| Duration of TKI therapy, y | 7.0 [3.0-17.6] |
| First-line treatment | |
| Imatinib | 89 (77.4) |
| 2G-TKI | 17 (14.8) |
| Other (interferon/autograft) | 9 (7.8) |
| Therapy ceased | |
| Imatinib | 70 (60.9) |
| First-line 2G-TKI, nilotinib/dasatinib, or 2G-TKI for imatinib-intolerance | 37 (32.2) |
| 2G-TKI following imatinib failure | 8 (7.0) |
| Baseline BCR-ABL1IS value available | 111 (96.5) |
| EMR achieved | 98 (90.7) |
| 3-mo BCR-ABL1 value available | 108 (93.9) |
| BCR-ABL1IS >10% | 10 (9.3) |
| BCR-ABL1IS >1%-10% | 28 (25.9) |
| BCR-ABL1IS >0.1%-1% | 43 (39.8) |
| BCR-ABL1IS ≤0.1% | 27 (25.0) |
| 3-mo halving time able to be calculated | 102 (88.7) |
| Median halving time (d) | 13.95 [3.6-267.8] |
| 3-mo BCR-ABL1 fold-reduction able to be calculated | 102 (88.7) |
| Median 3-mo BCR-ABL1 fold-reduction | 125.5 [3.1-8700] |
| Months between baseline and 3-mo BCR-ABL1 value | 3.1 [2.0-3.9] |
| 12-mo BCR-ABL1 value available | 112 (97) |
| MMR achieved | 86 (76.8) |
| Patient characteristic . | Patients attempting TFR with ≥12-mo follow-up No. (%) or median [range] . |
|---|---|
| Male | 61 (53.0) |
| Age at TFR attempt, y | 60.9 [33.4-89.8] |
| Sokal score | |
| Low-risk (<0.8) | 49 (42.6) |
| Intermediate-risk (0.8-1.2) | 41 (35.7) |
| High-risk (>1.2) | 17 (14.8) |
| Not available | 8 (7.0) |
| ELTS score | |
| Low-risk (≤1.5680) | 72 (63.2) |
| Intermediate-risk (>1.5680-≤2.2185) | 26 (22.4) |
| High-risk (>2.2185) | 8 (6.4) |
| Not available | 9 (8.0) |
| Transcript type | |
| e14a2 | 51 (44.3) |
| e13a2 | 43 (37.4) |
| Both e14a2 and e13a2 | 20 (17.4) |
| e1a2 | 1 (0.9) |
| Time to MR4.5, y | 2.2 [0.2-13.2] |
| Duration of MR4.5, y | 3.3 [2.0-12.5] |
| Duration of TKI therapy, y | 7.0 [3.0-17.6] |
| First-line treatment | |
| Imatinib | 89 (77.4) |
| 2G-TKI | 17 (14.8) |
| Other (interferon/autograft) | 9 (7.8) |
| Therapy ceased | |
| Imatinib | 70 (60.9) |
| First-line 2G-TKI, nilotinib/dasatinib, or 2G-TKI for imatinib-intolerance | 37 (32.2) |
| 2G-TKI following imatinib failure | 8 (7.0) |
| Baseline BCR-ABL1IS value available | 111 (96.5) |
| EMR achieved | 98 (90.7) |
| 3-mo BCR-ABL1 value available | 108 (93.9) |
| BCR-ABL1IS >10% | 10 (9.3) |
| BCR-ABL1IS >1%-10% | 28 (25.9) |
| BCR-ABL1IS >0.1%-1% | 43 (39.8) |
| BCR-ABL1IS ≤0.1% | 27 (25.0) |
| 3-mo halving time able to be calculated | 102 (88.7) |
| Median halving time (d) | 13.95 [3.6-267.8] |
| 3-mo BCR-ABL1 fold-reduction able to be calculated | 102 (88.7) |
| Median 3-mo BCR-ABL1 fold-reduction | 125.5 [3.1-8700] |
| Months between baseline and 3-mo BCR-ABL1 value | 3.1 [2.0-3.9] |
| 12-mo BCR-ABL1 value available | 112 (97) |
| MMR achieved | 86 (76.8) |
EMR, early molecular response; ELTS, EUTOS long-term survival.
Statistical analysis
Patient characteristics were described using frequencies and percentages for categorical variables, whereas continuous baseline measures were described using medians and interquartile ranges. The end point was the binary outcome of sustained TFR at 12 months. The secondary objective was molecular recurrence-free survival, defined as the time (in months) between cessation of TKI therapy and the first measurement of loss of MMR. Time-to-event modeling assessed the prognostic value of selected factors for molecular relapse. Time intervals (in either days or months) were entered into the analysis as continuous covariates. A series of univariate Cox proportional hazards regression models assessed and compared the prognostic value of each factor. Multivariate Cox proportional hazards regression modeling was performed on variables with a P value ≤ .1 on univariate analysis. Additional statistical tests to depict covariate significance include analysis of variance and unpaired t tests. A series of logistic regression analyses were used for predictive modeling of sustained TFR at 12 months using the final variables identified in the multivariate analysis. For the total CP-CML cohort, cumulative incidence graphs were generated to investigate the predictors of milestone achievement with the competing risks of allogeneic stem cell transplant, progression to blast crisis or death. Statistical analysis was performed using GraphPad Prism 8.0.0 and R version 3.6.1.
Results
Patient characteristics
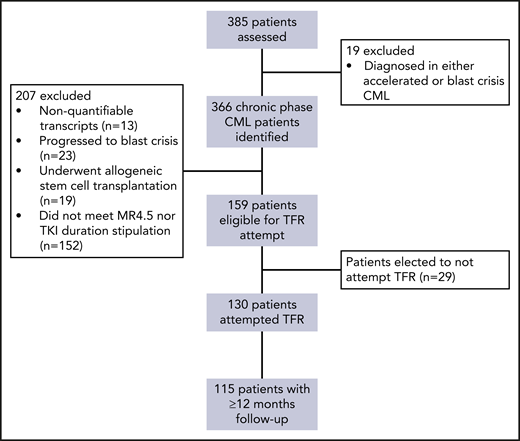
Of the total cohort of 385 South Australian patients, 366 patients diagnosed in CP-CML were monitored during the defined observational period (Figure 1). In total, 130 patients (36%) attempted TFR and met our specified eligibility criteria, of whom 115 (88%) had ≥12 months of follow-up following TKI discontinuation. Table 1 summarizes the patient characteristics for the 115 patients with ≥12 months of follow-up and supplemental Table 1 for the total 130 patients.
For the 115 TFR attempts with ≥12 months of follow-up, the median duration of follow-up after TKI cessation was 51.1 months (range, 12.0-153.2). Males accounted for 53% of patients. All but 1 patient had a p210 transcript. The remaining patient had an e1a2 transcript, which was also monitored using quantitative PCR. The majority of patients (61%) ceased imatinib and 39% stopped a 2G-TKI. Of the 45 patients stopping 2G-TKIs, 17 (38%) were treated first-line, whereas 20 (44%) switched for imatinib intolerance and 8 (18%) for resistance/suboptimal response to imatinib. The median duration of TKI therapy before the TFR attempt was 7.0 years (range, 3.0-17.6) and the median duration of MR4.5 was 3.3 years (range, 2.0-12.5).
Outcomes of TFR attempts
Of the 130 patients who ceased TKI, 54% (n = 70) remained off therapy at the last follow-up (supplemental Figure 2). The restricted mean time to molecular recurrence was 5.4 months (range, 1.4-33.4). Of the 115 patients with ≥12 months of follow-up after TKI cessation, 55% (n = 63) sustained TFR at 12 months (Figure 2A). Loss of MMR triggered TKI restart in 87% (n = 45) of molecular relapses in the first 12 months. The remaining patients (n = 7) restarted TKI because of progressive loss of DMR according to the more stringent recommencement strategy in the CML8 study.5 All but 1 patient were predicted to lose MMR within 1 month based on the BCR-ABL1 doubling time.39 MMR was regained at a median of 2.0 months (range, 0.2-9.3) in all patients and 96% regained MR4.5 after a median of 4.2 months (range, 0.7-44.2). Six additional patients (5.2%) lost MR4.5 but remained in MMR at 12 months. Three of these patients eventually resumed TKI; 1 patient at 13 months for progressively rising BCR-ABL1 levels (CML8 patient), whereas 2 patients lost MMR at 26.3 and 33.4 months, respectively. These 2 patients were the only late relapses in the follow-up of our cohort (2% of the ≥12-month cohort). Two of the 6 patients who lost MR4.5 spontaneously regained stable MR4.5, whereas the remaining patient had a fluctuating BCR-ABL1 level below MMR at 36 months.
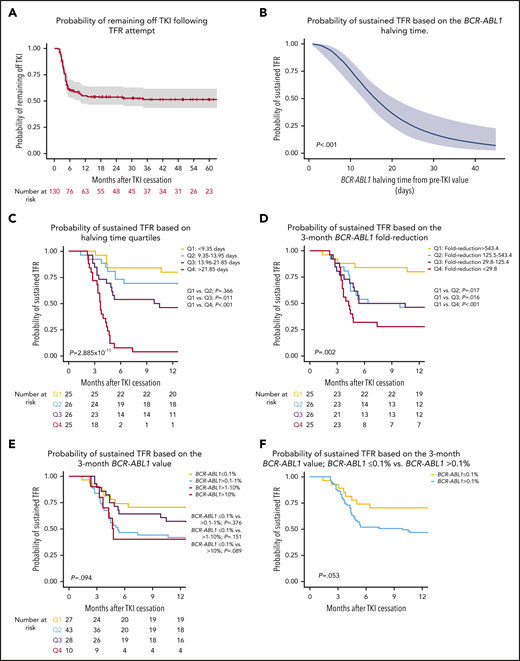
Probability of sustained TFR relative to halving time, 3-month BCR-ABL1 value and fold-reduction. (A) Kaplan-Meier analysis of probability of remaining off TKI in all TFR attempts with 95% confidence intervals shown. (B) Logistic regression analysis demonstrating the probability of sustained TFR based on individual halving times demonstrating that the likelihood of TFR is reduced with longer halving times. (C) Kaplan-Meier analysis of sustained TFR based on the BCR-ABL1 halving time quartiles. (D) Kaplan-Meier analysis of sustained TFR based on the fold-reduction of BCR-ABL1 from baseline. Patients were divided into quartile values. (E) Kaplan-Meier analysis of sustained TFR based on the 3-month BCR-ABL1 value. Comparison of BCR-ABL1IS ≤ 0.1% vs other groups shown. (F) Kaplan-Meier analysis of sustained TFR based on whether patients achieved a BCR-ABL1IS ≤0.1% at 3 months.
Probability of sustained TFR relative to halving time, 3-month BCR-ABL1 value and fold-reduction. (A) Kaplan-Meier analysis of probability of remaining off TKI in all TFR attempts with 95% confidence intervals shown. (B) Logistic regression analysis demonstrating the probability of sustained TFR based on individual halving times demonstrating that the likelihood of TFR is reduced with longer halving times. (C) Kaplan-Meier analysis of sustained TFR based on the BCR-ABL1 halving time quartiles. (D) Kaplan-Meier analysis of sustained TFR based on the fold-reduction of BCR-ABL1 from baseline. Patients were divided into quartile values. (E) Kaplan-Meier analysis of sustained TFR based on the 3-month BCR-ABL1 value. Comparison of BCR-ABL1IS ≤ 0.1% vs other groups shown. (F) Kaplan-Meier analysis of sustained TFR based on whether patients achieved a BCR-ABL1IS ≤0.1% at 3 months.
Predictors of TFR outcome
The candidate prognostic variables shown in Table 1 were investigated for association with sustained TFR: sex, age at TFR attempt, Sokal and ELTS42 scores, transcript type, time to MR4.5, duration of MR4.5, total duration of TKI therapy, TKI at TFR attempt, first-line TKI, first-line vs second-line TKI, EMR, baseline and 3-month BCR-ABL1 value, 3-month BCR-ABL1 fold-reduction, 3-month BCR-ABL1 halving time, and MMR at 12 months of TKI therapy.
Univariate Cox regression analysis for prediction of sustained TFR at 12 months is shown in Table 2. Variables demonstrating a significant association on univariate analysis were halving time (Figure 2B-C), transcript type, BCR-ABL1 fold-reduction (Figure 2D), and 3-month BCR-ABL1IS value (Figure 2E-F). Variables with a P value ≤0.1 were included in the multivariate Cox regression analysis. Halving time and transcript type remained significant predictors of sustained TFR. Duration of TKI exposure was also an independent predictor (Table 2). A second multivariate analysis was performed using the fold-reduction value from baseline to 3 months, excluding halving time to avoid collinearity. Fold-reduction, transcript type, and duration of TKI exposure were independent predictors of sustained TFR (supplemental Table 2). However, when comparing the concordance-index of the 2 models, the analysis including halving time had a higher value, implying that this model has slightly better discriminatory power for predicting sustained TFR.
Univariate and multivariate Cox regression analysis of variables investigated as potential predictors of sustained TFR at 12 mo
| Variable . | No. of patients (%) . | Patients in sustained TFR (%) . | Univariate analysis . | Multivariate analysis . | |||||
|---|---|---|---|---|---|---|---|---|---|
| β-coefficient . | HR (95% CI) . | P . | β-coefficient . | HR (95% CI) . | P . | Concordance index§ . | |||
| Halving time, d* | 102 (88.7) | 0.02 | 1.02 (1.01-1.03) | 2.26 × 10−8 | 0.03 | 1.03 (1.02-1.05) | 1.91 × 10−6 | 0.745 | |
| Transcript | |||||||||
| e14a2 | 51 (44.3) | 34 (66.7) | Ref | — | — | Ref | — | — | |
| e13a2 | 43 (37.4) | 17 (39.5) | 0.88 | 2.41 (1.30-4.44) | .005 | 0.91 | 2.49 (1.39-4.46) | .002 | |
| Both transcripts | 20 (17.4) | 11 (55.0) | 0.33 | 1.39 (0.62-3.11) | .43 | — | — | — | |
| Duration of TKI therapy, y* | 115 (100) | 0.56 | 1.75 (0.92-3.33) | .09 | 0.95 | 2.61 (1.05-6.09) | .03 | ||
| Time to MR4.5, y* | 115 (100) | −0.08 | 1.08 (0.99-1.18) | .08 | −0.07 | 0.93 (0.86-1.06) | .26 | ||
| 3-mo BCR-ABL1IS value* | 108 (94) | 0.03 | 1.03 (1.00-1.05) | .03 | −0.004 | 1.00 (0.96-1.03) | .80 | ||
| 3-mo BCR-ABL1 fold-reduction† | 102 (88.7) | 2.8 × 10−6 | 1.00 (1.00-1.01) | .008 | |||||
| Baseline BCR-ABL1IS value* | 111 (96.5) | −0.002 | 0.99 (0.99-1.00) | .35 | |||||
| Duration of MR4.5, y* | 115 (100) | 0.29 | 1.33 (0.75-2.38) | .33 | |||||
| Sex | |||||||||
| Male | 61 (53.0) | 32 (52.5) | Ref | — | — | ||||
| Female | 54 (47.0) | 31(57.4) | −0.16 | 0.85 (0.49-1.47) | .57 | ||||
| Age at TFR attempt* | 115 (100) | 0.26 | 1.30 (0.76-2.25) | .34 | |||||
| Sokal score | |||||||||
| Low | 49 (45.8) | 25 (51.0) | Ref | — | — | ||||
| Intermediate | 41 (38.3) | 22 (53.7) | −0.10 | 0.91 (0.50-1.65) | .74 | ||||
| High | 17 (15.9) | 9 (52.9) | <0.01 | 1.01 (0.45-2.24) | .99 | ||||
| ELTS score | |||||||||
| Low | 72 (67.9) | 38 (52.8) | Ref | — | — | ||||
| Intermediate | 26 (24.5) | 11 (42.3) | 0.28 | 1.33 (0.72-2.44) | .36 | ||||
| High | 8 (7.5) | 7 (87.5) | −1.57 | 0.20 (0.03-1.52) | .12 | ||||
| TKI ceased | |||||||||
| First-line imatinib | 60 (52.2) | 40 (66.7) | Ref | — | — | ||||
| First-line 2G-TKI | 17 (14.8) | 11 (64.7) | 0.26 | 1.29 (0.73-2.30) | .37 | ||||
| 2G-TKI for imatinib resistance | 8 (7.0) | 6 (75.0) | −0.09 | 0.92 (0.28-3.02) | .89 | ||||
| First-line TKI | |||||||||
| Imatinib | 89 (77.4) | 51 (57.3) | Ref | — | — | ||||
| 2G-TKI | 17 (14.8) | 12 (70.6) | −0.68 | 0.47 (0.20-1.27) | .14 | ||||
| EMR achieved | 98 (85.2) | 53 (54.1) | −0.38 | 0.68 (0.29-1.61) | .38 | ||||
| MMR by 12 mo | 86 (76.8) | 46 (53.5) | 0.09 | 1.10 (0.57-2.10) | .78 | ||||
| Variable . | No. of patients (%) . | Patients in sustained TFR (%) . | Univariate analysis . | Multivariate analysis . | |||||
|---|---|---|---|---|---|---|---|---|---|
| β-coefficient . | HR (95% CI) . | P . | β-coefficient . | HR (95% CI) . | P . | Concordance index§ . | |||
| Halving time, d* | 102 (88.7) | 0.02 | 1.02 (1.01-1.03) | 2.26 × 10−8 | 0.03 | 1.03 (1.02-1.05) | 1.91 × 10−6 | 0.745 | |
| Transcript | |||||||||
| e14a2 | 51 (44.3) | 34 (66.7) | Ref | — | — | Ref | — | — | |
| e13a2 | 43 (37.4) | 17 (39.5) | 0.88 | 2.41 (1.30-4.44) | .005 | 0.91 | 2.49 (1.39-4.46) | .002 | |
| Both transcripts | 20 (17.4) | 11 (55.0) | 0.33 | 1.39 (0.62-3.11) | .43 | — | — | — | |
| Duration of TKI therapy, y* | 115 (100) | 0.56 | 1.75 (0.92-3.33) | .09 | 0.95 | 2.61 (1.05-6.09) | .03 | ||
| Time to MR4.5, y* | 115 (100) | −0.08 | 1.08 (0.99-1.18) | .08 | −0.07 | 0.93 (0.86-1.06) | .26 | ||
| 3-mo BCR-ABL1IS value* | 108 (94) | 0.03 | 1.03 (1.00-1.05) | .03 | −0.004 | 1.00 (0.96-1.03) | .80 | ||
| 3-mo BCR-ABL1 fold-reduction† | 102 (88.7) | 2.8 × 10−6 | 1.00 (1.00-1.01) | .008 | |||||
| Baseline BCR-ABL1IS value* | 111 (96.5) | −0.002 | 0.99 (0.99-1.00) | .35 | |||||
| Duration of MR4.5, y* | 115 (100) | 0.29 | 1.33 (0.75-2.38) | .33 | |||||
| Sex | |||||||||
| Male | 61 (53.0) | 32 (52.5) | Ref | — | — | ||||
| Female | 54 (47.0) | 31(57.4) | −0.16 | 0.85 (0.49-1.47) | .57 | ||||
| Age at TFR attempt* | 115 (100) | 0.26 | 1.30 (0.76-2.25) | .34 | |||||
| Sokal score | |||||||||
| Low | 49 (45.8) | 25 (51.0) | Ref | — | — | ||||
| Intermediate | 41 (38.3) | 22 (53.7) | −0.10 | 0.91 (0.50-1.65) | .74 | ||||
| High | 17 (15.9) | 9 (52.9) | <0.01 | 1.01 (0.45-2.24) | .99 | ||||
| ELTS score | |||||||||
| Low | 72 (67.9) | 38 (52.8) | Ref | — | — | ||||
| Intermediate | 26 (24.5) | 11 (42.3) | 0.28 | 1.33 (0.72-2.44) | .36 | ||||
| High | 8 (7.5) | 7 (87.5) | −1.57 | 0.20 (0.03-1.52) | .12 | ||||
| TKI ceased | |||||||||
| First-line imatinib | 60 (52.2) | 40 (66.7) | Ref | — | — | ||||
| First-line 2G-TKI | 17 (14.8) | 11 (64.7) | 0.26 | 1.29 (0.73-2.30) | .37 | ||||
| 2G-TKI for imatinib resistance | 8 (7.0) | 6 (75.0) | −0.09 | 0.92 (0.28-3.02) | .89 | ||||
| First-line TKI | |||||||||
| Imatinib | 89 (77.4) | 51 (57.3) | Ref | — | — | ||||
| 2G-TKI | 17 (14.8) | 12 (70.6) | −0.68 | 0.47 (0.20-1.27) | .14 | ||||
| EMR achieved | 98 (85.2) | 53 (54.1) | −0.38 | 0.68 (0.29-1.61) | .38 | ||||
| MMR by 12 mo | 86 (76.8) | 46 (53.5) | 0.09 | 1.10 (0.57-2.10) | .78 | ||||
CI, confidence interval; HR, hazard ratio; Ref, reference variable.
Continuous variables.
Harrell’s Concordance Index is a measure of the goodness of fit of the prediction model.58
3-month fold-reduction is assessed in a separate multivariate analysis model to avoid collinearity with the 3-mo halving time (supplemental Table 2).
Of the 115 patients who attempted TFR with ≥12 months of follow-up after TKI discontinuation, BCR-ABL1 halving times28 could be calculated in 102 (89%). The remaining 11% either did not commence TKI as first-line treatment (n = 9) or the baseline and/or the 3-month BCR-ABL1 value were unavailable. Patients with a sustained TFR had a shorter median halving time (10.1 days; range, 3.6-26.4) compared with patients with molecular relapse (21.7 days; range, 7.1-267.8), P < .001 (supplemental Figure 3). Logistic regression also confirmed that the likelihood of sustained TFR at 12 months was lower in patients with longer halving times (Figure 2B). For example, patients with a halving time of 10 days who attempted TFR once eligible had almost 75% probability of sustained TFR, falling to ∼12% for patients with a halving time of 40 days. Patients were divided into quartiles based on halving time: the probability of sustained TFR was 80% in patients in the first quartile (Q1, halving time <9.35 days) compared with 4% in patients in the fourth quartile (Q4, halving time >21.85 days), P < .001 (Figure 2C). The late relapses had halving times in the third and fourth quartiles with gradually rising BCR-ABL1 values until eventual molecular relapse (supplemental Figure 2). All patients with a halving time in Q4 (>21.85 days) eventually experienced molecular relapse during follow-up. Interestingly, there was an association between the BCR-ABL1 doubling time at molecular relapse and the initial halving time, such that patients with the shortest halving time (Q1) had longer median doubling times compared with patients in Q2 through Q4 (supplemental Figure 4). Division of patients based on the BCR-ABL1 fold-reduction confirmed that a more rapid BCR-ABL1 decline (ie, Q1: >543.4 fold-reduction) was associated with an 80% probability of sustained TFR (Figure 2D). However, patients with a fold-reduction of <29.8 (Q4) still had a 28% probability of sustained TFR, P = .002. Furthermore, we were also able to demonstrate that unlike the patients with a Q4: halving time, patients with a Q4: fold-reduction were able to remain in long-term TFR (supplemental Figure 5), indicating that the BCR-ABL1 halving time has better discriminatory power for prediction of sustained TFR compared with the fold-reduction.
Because the BCR-ABL1 halving time predicted sustained TFR, the 3-month BCR-ABL1IS value was assessed for similar predictive power using Kaplan-Meier analysis (Figure 2E). Patients with a 3-month BCR-ABL1IS of ≤0.1% had a 70% probability of sustained TFR compared with 40% for patients with a BCR-ABL1IS of >10%, P = .089. Splitting patients further into those with and without a BCR-ABL1IS value of ≤0.1% at 3 months (Figure 2F) approached statistical significance (P = .053). However, the 3-month BCR-ABL1 value was not a significant predictor of sustained TFR on multivariate analysis (Table 2). This confirmed that the 3-month BCR-ABL1 value is not interchangeable with the halving time as a predictor of sustained TFR.
The estimated proportion of e14a2 transcript patients in sustained TFR was 67% compared with 40% for e13a2 patients (P = .005). The simultaneous expression of both transcripts did not predict sustained TFR (Table 2). Additionally, there was no association between the halving time and transcript type (supplemental Figure 6A). However, for patients with either e14a2 or e13a2 transcript types, the halving times of patients in sustained TFR (supplemental Figure 6B-C) were shorter than those with molecular relapse. Patients with the e14a2 transcript and sustained TFR had a median halving time of 10.2 vs 22.3 days for those with molecular relapse (P = .01). Similarly, patients with the e13a2 transcript and sustained TFR had a median halving time of 10.7 vs 21.5 days for those with molecular relapse (P = .012).
Validation of halving time as a predictor of TFR outcome
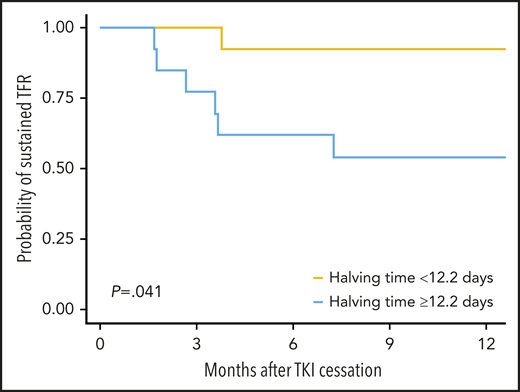
Independent validation of the initial BCR-ABL1 halving time as a predictor of sustained TFR was performed using an external dataset of 26 first-line TKI treated patients attempting TFR in Korea. The characteristics are detailed in supplemental Table 3. This dataset involved a combination of patients enrolled in the KID study9 (n = 19) and local registries (n = 7). The rate of sustained TFR at 12 months was 73%. The median BCR-ABL1 halving time of all 26 patients was 12.2 days (range, 6.0-35.0). Halving time was validated as a predictor of sustained TFR (Figure 3). Patients with halving time below the median of 12.2 days had a 92% probability of sustained TFR, whereas patients with longer halving times had a 54% probability of sustained TFR (P = .041).
Probability of sustained TFR in the validation cohort based on the median halving time.
Probability of sustained TFR in the validation cohort based on the median halving time.
Prediction of TFR eligibility
We assessed the initial BCR-ABL1 halving time, the fold-reduction of BCR-ABL1 from baseline to 3 months and the 3-month BCR-ABL1 value for prediction of eligibility for a TFR attempt. All CP-CML patients with available halving times (n = 298) were divided into the same quartile groups that were derived from the cohort attempting TFR with 12 months of follow-up (Figure 4A). Cumulative incidence analysis stratified by halving time demonstrated that by 5 years of TKI therapy, 71% of patients in Q1 (halving time <9.35 days) achieved TFR eligibility, vs only 8.9% of Q4 (halving time >21.85 days), P < .001. Logistic regression analysis of halving time at specific durations (5 and 8 years) of TKI therapy confirmed that shorter halving times remained predictive of TFR eligibility (supplemental Figures 7 and 8). For example, a halving time of 10 days predicted for ∼50% probability of TFR eligibility by 5 years, increasing to ∼56% by 8 years. Patients with a halving time of 40 days had a 25% probability of TFR eligibility by 5 years, increasing to only ∼35% by 8 years of TKI therapy. Similarly, cumulative incidence analysis demonstrated that the BCR-ABL1 fold-reduction predicted for TFR eligibility (Figure 4B). Confirming our previous published work (14.8% patient overlap),26 the 3-month BCR-ABL1 value predicted TFR eligibility in 309 patients with available 3-month BCR-ABL1 values (P < .001; Figure 4C). This analysis demonstrates that the BCR-ABL1 response at 3 months of first-line TKI is a powerful predictor of TFR eligibility, whether measured as the rate of BCR-ABL1 decline from baseline or by assessing the actual BCR-ABL1 value. However, the 3-month value on its own did not predict sustained TFR.
Prediction of TFR eligibility in the total CP-CML cohort of 366 patients. (A) Cumulative incidence of TFR eligibility based on halving time quartiles. (B) Cumulative incidence of TFR eligibility based on the BCR-ABL1 fold-reduction. (C) Cumulative incidence of TFR eligibility based on the 3-month BCR-ABL1 value.
Prediction of TFR eligibility in the total CP-CML cohort of 366 patients. (A) Cumulative incidence of TFR eligibility based on halving time quartiles. (B) Cumulative incidence of TFR eligibility based on the BCR-ABL1 fold-reduction. (C) Cumulative incidence of TFR eligibility based on the 3-month BCR-ABL1 value.
Modeling sustained TFR in all chronic phase CML patients
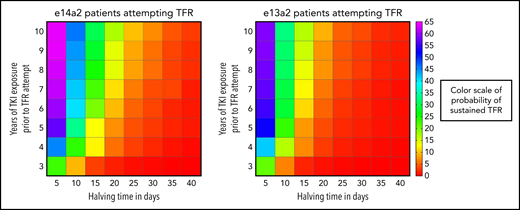
We modeled prediction of sustained TFR in all CP-CML patients based on the strongest combination of variables that emerged from the multivariate analysis: halving time, transcript type, and duration of TKI exposure. A series of logistic regression analyses was performed to calculate the probability of TFR eligibility and sustained TFR based on these variables. These calculations were combined to develop a composite prediction model (Figure 5). Using the model, which assumes that only eligible patients attempt TFR, clinicians can predict the probability of sustained TFR based on halving time, years of TKI exposure and transcript type. For example, a patient with an e14a2 transcript and an initial BCR-ABL1 halving time of 5 days has a 30% probability of sustained TFR, if attempted after 3 years of TKI exposure. This increases to ∼65% if the TFR attempt is deferred to 8 years of TKI exposure. In contrast, patients with a slower initial BCR-ABL1 decline (halving time ≥35 days) have <5% chance of attaining a sustained TFR irrespective of the transcript type and length of TKI exposure. This model could be useful for patient counseling before TFR attempts.
Personalized prediction model for sustained TFR based on halving time (days), transcript type, and duration of TKI exposure (years). The color scale to predict the probability of sustained TFR is depicted on the right side of the figure and represents the percent probability. Patients were divided into the major transcript groups e14a2 and e13a2. The vertical axis is the years of TKI exposure before TFR attempt and the horizontal axis is the halving time.
Personalized prediction model for sustained TFR based on halving time (days), transcript type, and duration of TKI exposure (years). The color scale to predict the probability of sustained TFR is depicted on the right side of the figure and represents the percent probability. Patients were divided into the major transcript groups e14a2 and e13a2. The vertical axis is the years of TKI exposure before TFR attempt and the horizontal axis is the halving time.
Discussion
We have shown that a rapid initial decline in BCR-ABL1 transcripts for first-line TKI-treated patients is an independent predictor of TFR eligibility and subsequent sustained TFR. BCR-ABL1 transcript type and duration of TKI therapy before the TFR attempt were also independent predictors of sustained TFR. Duration of MR4.5 was not a predictor of sustained TFR in our data compared with the Euro-SKI study.4 This likely reflects our proactive approach to TFR attempts whereby many patients promptly ceased TKI shortly after attaining TFR eligibility. A model including BCR-ABL1 halving time measured at 3 months had greater discriminatory power to predict sustained TFR than the fold-reduction of BCR-ABL1 at 3 months. We also demonstrate that no patients with long halving times (Q4) remained in long-term TFR (supplemental Figure 2), whereas fold-reduction, outside of Q1, is less able to differentiate patients for long-term TFR (supplemental Figure 5). This strengthens our argument that although fold-reduction can predict TFR outcomes, halving time is a far better discriminator, presumably because it more accurately reflects the true rate of leukemic decline by incorporating the number of days between the start of TKI therapy and the day of BCR-ABL1 measurement,28 which varied by almost 60 days in our cohort for the 3-month time point (Table 1).
Importantly, we were able to validate the association between the initial rate of BCR-ABL1 decline and sustained TFR in an independent dataset. Such datasets with available BCR-ABL1 values measured at diagnosis and at 3 months of first-line TKI using internationally standardized quantitative methods are not readily available. This relates to the long duration of therapy before attempting TFR in most studies. Many patients enrolled in cessation studies were from regional centers where molecular monitoring was not performed or the patient was diagnosed before methods were standardized and in routine use. Furthermore, quantification of BCR-ABL1 transcripts at diagnosis is not routinely performed for all patients and is not mandated by the European LeukemiaNet.3 We suggest that quantification of BCR-ABL1 transcripts at diagnosis is essential for outcome prediction and should be incorporated into the monitoring strategy.
Shorter halving times or a greater BCR-ABL1 fold-reduction indicate a more rapid decline in measurable leukemia after commencing TKI, likely reflecting both the intensity of kinase inhibition and the individual sensitivity of the leukemic cells. Our data are consistent with the concept that the initial rate of BCR-ABL1 decline has a stronger correlation with the degree of suppression of the leukemic clone than an absolute BCR-ABL1 value at a predetermined time point. Whether strategies targeting a rapid initial BCR-ABL1 decline using more potent TKIs will translate into a higher overall probability of sustained TFR is yet to be determined. Decisions about first-line therapy must always be made in the context of the patient’s comorbidities because 2G-TKIs are associated with significantly higher rates of serious toxicity.12,13,43 Dedicated 2G-TKI TFR studies addressing the rapidity of the initial BCR-ABL1 response will be required to investigate this hypothesis further before incorporating considerations of halving times into treatment recommendations.
We developed a prediction model for sustained TFR comprising the strongest combination of variables (halving time, transcript type, and duration of TKI exposure; Figure 5). This could potentially be used when counseling patients about the potential for sustained TFR. However, this model was developed from a population that was predominantly treated with first-line imatinib and refinement will likely occur with data from more first-line 2G-TKI-treated patients.
The impact of transcript type on CML outcomes has long been debated.44-46 The e14a2 transcript and the shorter e13a2 transcript encode protein products that differ by 25 amino acids.44,47 Superior molecular responses have been reported in patients with e14a2 transcripts, with more rapid achievement of molecular milestones such as MMR and MR4.5 on imatinib treatment,48 although the differences were less pronounced with 2G-TKI therapy.44 Overall, survival outcomes are largely similar between the transcript groups.44,46,48,49 Claudiani et al18 provided the first report that the e14a2 transcript was associated with a higher rate of sustained TFR in a retrospective analysis of 64 patients attempting TFR. In our larger cohort, we observed that the e14a2 transcript type is an independent predictor of sustained TFR. Additionally, the effect of transcript type on sustained TFR is independent of halving time (supplemental Figure 6). Although the exact mechanism for superior responses in e14a2 patients is not understood, the transcript is predicted to be more immunogenic,50,51 leading to increased immune-mediated clearance, perhaps contributing to the higher probability of sustained TFR.52
The control gene in our assay is BCR and the decline of BCR-ABL1 relative to BCR has been shown to be exponential, ratifying the halving time formula used in this work.28 However, the use of an ABL1-based assay is more widespread and our validation data were derived from an ABL1-based method. A number of groups have now established the reliability of ABL1-obtained BCR-ABL1 values in halving time calculations.30-34,53,54 Use of the halving time formula based on values derived from BCR-ABL1 assays with alternative control genes such as GUSB54 and GAPDH35 has also been published. We have demonstrated a significant association between halving time, TFR eligibility, and sustained TFR, but it is essential that the initial BCR-ABL1 decline demonstrates an exponential response for the halving time formula to be valid, regardless of the control gene used.28
TFR attempts remain extremely safe, although blast crisis is a rare event that has been reported in <1% of patients.6,55-57 In our analysis, no patient who attempted TFR has progressed to blast crisis with up to 153.2 months of follow-up. The timing of blast crisis in the few reported cases was variable and in a single instance, occurred months after TKI recommencement despite reachievement of MMR.6 Hence, although there is no clear predictor as yet for the development of blast crisis in patients attempting TFR, clinicians should remain vigilant for this rare complication by continued regular monitoring.
In conclusion, we have demonstrated that the initial rate of BCR-ABL1 decline, specifically measured as halving time, is a powerful predictor of sustained TFR. Understanding the extrinsic determinants and the intrinsic biological factors that influence the rate of leukemic decline will be imperative to maximize the number of patients that can successfully attempt TFR. A model incorporating the initial rate of BCR-ABL1 decline and other independent predictors of sustained TFR will likely enhance long-term clinical management decisions for clinicians and patients.
Deidentified participant data collected for our study can be made available to researchers once appropriate ethical approval and a signed data access agreement is obtained.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Jana Bednarz from the University of Adelaide for assistance with the statistical methods included in the manuscript.
This study was supported by funding from the Royal Adelaide Hospital Research Foundation Dawes Scholarship (N.S.), the National Health and Medical Research Council of Australia (APP1117718) (S.B.), the National Health and Medical Research Council of Australia (APP1135949) (T.P.H.), and the Cancer Council SA’s Beat Cancer Project (T.P.H.).
Authorship
Contribution: N.S. collected and analyzed the data and wrote the paper; I.S.P. provided key insights into the paper and reviewed the manuscript; D.T.Y., D.K.H., A.S.M.Y., and D.M.R. reviewed the manuscript; J.A.B. and H.K.A. performed the molecular analysis and reviewed the manuscript; S.P. and D.-W.K. contributed data and reviewed the paper; S.B. contributed key concepts and methodology and reviewed the manuscript; and T.P.H. conceptualized the paper and reviewed the manuscript.
Conflict-of-interest disclosure: N.S. received honoraria from Novartis and travel and accommodation expenses from Novartis, Gilead, Amgen, and Janssen; S.B. is a member of the advisory boards of Qiagen, Novartis, and Cepheid, received honoraria from Qiagen, Novartis, Bristol-Myers Squibb, Incyte, and Cepheid, and research funding from Novartis; A.S.M.Y. received research funding from Novartis, BMS, and Celgene and honoraria from Novartis and BMS; D.M.R. received research funding and honoraria from Novartis and BMS; T.P.H. is a member of advisory boards and has received research funding and honoraria from Novartis and Bristol-Myers Squibb;. D.-W.K. received research funding and honoraria from Novartis, Bristol-Myers Squibb, Otsuka, Takeda, Sun Pharma, and ILYANG. The remaining authors declare no competing financial interests.
Correspondence: Naranie Shanmuganathan, Precision Medicine Theme, South Australian Health & Medical Research Institute (SAHMRI) and Centre for Cancer Biology, SA Pathology, North Terrace, Adelaide, SA 5000, Australia; e-mail: naranie.shanmuganathan@sa.gov.au.
REFERENCES
Author notes
S.B. and T.P.H. are equal senior authors.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal