Key Points
In medically fit patients with CLL, a fixed-duration (15-month) approach achieved a persistent MRD benefit beyond the end of treatment.
The fixed-duration approach was associated with high 3-year progression-free and overall survival rates and low long-term toxicity.
Abstract
Trials assessing first-line, fixed-duration approaches in chronic lymphocytic leukemia (CLL) are yielding promising activity, but few long-term data are available. We report follow-up data from a phase 2 trial (ICLL07 FILO) in previously untreated, medically fit patients (N = 135). Patients underwent obinutuzumab-ibrutinib induction for 9 months; then, following evaluation (N = 130 evaluable), those in complete remission and with bone marrow measurable residual disease (BM MRD) <0.01% (n = 10) received ibrutinib for 6 additional months; those in partial remission and/or with BM MRD ≥0.01%, the majority (n = 120), also received 4 cycles of immunochemotherapy (fludarabine/cyclophosphamide-obinutuzumab). Beyond end of treatment, responses were assessed every 3 month and peripheral blood MRD every 6 months. At median follow-up 36.7 months from treatment start, progression-free and overall survival rates (95% confidence interval) at 3 years were 95.7% (92.0% to 99.5%) and 98% (95.1% to 100%), respectively. Peripheral blood MRD <0.01% rates were 97%, 96%, 90%, 84%, and 89% at months 16, 22, 28, 34, and 40, respectively. No new treatment-related or serious adverse event occurred beyond end of treatment. Thus, in previously untreated, medically fit patients with CLL, a fixed-duration (15 months), MRD-guided approach achieved high survival rates, a persistent MRD benefit beyond the end of treatment, and low long-term toxicity. This trial was registered at www.clinicaltrials.gov as #NCT02666898.
Introduction
The availability of targeted agents (ibrutinib, obinutuzumab, venetoclax) has led to interest in potentially sparing some patients with chronic lymphocytic leukemia (CLL) from the adverse effects of standard timmunochemotherapy, such as 6 cycles of fludarabine/cyclophosphamide (FC)/rituximab (FCR).1 Ibrutinib was initially developed for continuous regimens, but many patients discontinue treatment because of side effects or CLL progression.2-6 Recent research has focused on developing effective fixed-duration approaches, with targeted agents given either with chemotherapy or in combinations with each other.7-12
The phase 2 ICLL-07 trial (NCT02666898) from the French Innovative Leukemia Organization (FILO) evaluated a fixed-duration, measurable residual disease (MRD)-guided approach in previously untreated, medically fit patients with CLL.13 Patients underwent obinutuzumab-ibrutinib induction for 9 months then an MRD-guided strategy for 6 additional months, in which those in complete remission (CR) with bone marrow (BM) MRD <0.01% received ibrutinib (10 of 130 evaluable); those in partial remission (PR) and/or with BM MRD ≥0.01%, the majority (120 of 130 evaluable), also received 4 cycles of immunochemotherapy (FC-obinutuzumab). At month 16, 62% (90% confidence interval [CI], 55% to 69%) of all patients enrolled (84 of 135) achieved the primary outcome of complete response with BM MRD <0.01%. This activity is higher than reported for immunochemotherapy regimens containing only rituximab or obinutuzumab as the targeted agent.14-20 Promising activity is also emerging for other first-line, fixed-duration approaches7-12 ; however, few long-term data are yet available.11,12
Here, we report follow-up data to further investigate the quality of responses to our experimental strategy, and explore long-term survival, the evolution of MRD beyond the end of treatment, and safety.
Study design
ICLL-07 study design (supplemental Figure 1, available on the Blood Web site), inclusion/exclusion criteria (supplemental Data file), and methodology are published.13 Between 27 October 2015 and 16 May 2017, 135 previously untreated, medically fit patients were enrolled from 27 FILO centers (supplemental Figure 2). All received 8 infusions of obinutuzumab 1000 mg over 6 × 4-weekly cycles plus oral ibrutinib 420 mg per day for 9 months. Then, those in CR with BM MRD <0.01% (10 of 130 evaluable) continued ibrutinib for 6 months; those in PR and/or with BM MRD ≥0.01% (120 of 130 evaluable) received 4 × 4–weekly cycles of IV FC and obinutuzumab 1000 mg alongside continuing ibrutinib. Beyond month 16, ibrutinib was stopped, except for patients in the FC-obinutuzumab plus ibrutinib arm in CR with BM MRD ≥0.01%, who continued ibrutinib until peripheral blood (PB) MRD reached <0.01%.
Responses at month 16 were assessed by investigators, according to International Working Group on CLL (iwCLL) 2008 guidelines.21 A subsequent blinded, centralized review included review of whole-body computed tomography (CT) scans, with the CR definition requiring no lymph nodes >1.5 cm in diameter. MRD was assessed oligocentrally (4 laboratories) on fresh BM (main study) and PB (main study and follow-up) samples by 8-color flow cytometry, using a technique developed by the FILO (PHYLLO) group.22 Classical MRD interpretation uses a threshold of 0.01%, per studies of 4-color assays.21 With our highly sensitive (10−6) technique, positivity criteria included isolation of 10 CLL cells representing at least a rate of 0.00003%. This enabled subdivision of MRD <0.01% into low-level positive MRD <0.01% and undetectable MRD.22
Beyond month 16, for 3 years, patients were followed for response every 3 months, progression-free survival (PFS), overall survival (OS), PB MRD every 6 months, and safety.
Data cutoff was 4 May 2020, at median follow-up 36.7 months from treatment start. Statistical analyses used the Fisher exact test.
Results and discussion
In the ICLL-07 primary analysis at month 16, published by Michallet et al,13 the CR rate by investigator assessment was 73.3% (99 of 135) for all enrolled patients and 80.5% (99 of 123) for evaluable patients. The subsequent blinded, centralized review, including CT scan reviews, was performed for 109 patients (the remaining 13 patients had no available compact disc of CT scans) and confirmed the CR rate as 73.3% (80 of 109) (supplemental Figure 3). Twelve patients categorized as in CR by the investigators were recategorized as in PR, due to their CT scans showing lymph nodes >1.5 cm in diameter (all 1.6-2.0 cm). The BM MRD <0.01% rate in the primary analysis was 79.3% (107 of 135) for all enrolled patients and 94.7% (107 of 113) for evaluable patients. In the deeper MRD analysis, the evaluable BM MRD <0.01% rate could be subdivided into a low-level positive BM MRD <0.01% rate of 14.2% (16 of 113) and an undetectable BM MRD rate of 80.5% (91 of 113). These more stringent assessments further substantiate the high quality of activity achieved with our experimental strategy. Our high BM MRD <0.01% rate is substantiated by findings for a similar first-line, fixed-duration ibrutinib plus FC-obintuzumab regimen (3 cycles FC-obinutuzumab immunochemotherapy; then 3 cycles ibrutinib-obinutuzumab and 6 cycles ibrutinib if CR with BM MRD <0.01%, or 9 cycles ibrutinib-obinutuzumab otherwise) in medically fit patients with IGHV-mutated status, where 41 of 45 enrolled patients completed 12 cycles of treatment and reached BM MRD <0.01%.12 Reported BM MRD <0.01% rates for other fixed-duration approaches include 84% for 6 cycles of FCR plus 2 years of ibrutinib in younger patients,7 57% for 12 cycles of venetoclax-obinutuzumab in patients with coexisting conditions,11 and 79% with 24 cycles of venetoclax-ibrutinib in high-risk/older patients.8
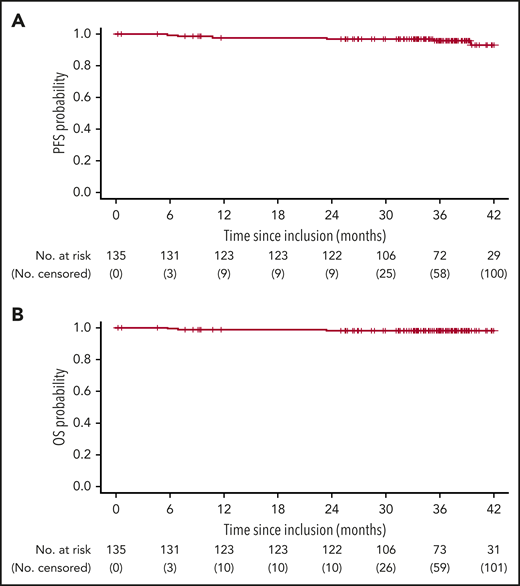
Three-year PFS and OS rates (95% CI) in our study, at 20 months from end of treatment, were 95.7% (92.0% to 99.5%) and 97.7% (95.1% to 100%), respectively (Figure 1), demonstrating prolonged remission after stopping treatment, including ibrutinib. In the similar trial evaluating a 12-cycle ibrutinib plus FC-obinutuzumab regimen, no patient had a CLL progression at median follow-up 18.7 months beyond end of treatment.12 These data are relevant because in a trial of 6 cycles of ibrutinib-rituximab followed by continuous ibrutinib in younger patients, among those who discontinued ibrutinib for toxicity or other nonprogression/death reason, the median time from discontinuation to disease progression/death was only 23 months.23 A 2-year PFS rate, at 1 year from end of treatment, of 88% (95% CI, 84% to 93%) was reported for 12 cycles of venetoclax-obinutuzumab in patients with coexisting conditions.11
Survival outcomes in all evaluable patients. (A) PFS (6 events). (B) OS (3 events). Three died without progression before death (accidental fall at month 7; unexpected sudden cardiac death with no autopsy at month 8 (treatment relationship unknown; Hodgkin lymphoma Epstein-Barr virus–associated hemophagocytosis at month 23). Three further patients experienced progression (at months 11, 35, and 39). Following progression, patients could continue to be followed for OS. After 42 months, follow-up for survival outcomes was censored due to the low numbers of patients at risk. One clinical relapse occurred at month 38, 22 months after the end of treatment; the patient had CR (confirmed by blinded review) with undetectable MRD at month 16, and concomitantly initiated surgical and radiotherapy for epidermoid gum cancer.
Survival outcomes in all evaluable patients. (A) PFS (6 events). (B) OS (3 events). Three died without progression before death (accidental fall at month 7; unexpected sudden cardiac death with no autopsy at month 8 (treatment relationship unknown; Hodgkin lymphoma Epstein-Barr virus–associated hemophagocytosis at month 23). Three further patients experienced progression (at months 11, 35, and 39). Following progression, patients could continue to be followed for OS. After 42 months, follow-up for survival outcomes was censored due to the low numbers of patients at risk. One clinical relapse occurred at month 38, 22 months after the end of treatment; the patient had CR (confirmed by blinded review) with undetectable MRD at month 16, and concomitantly initiated surgical and radiotherapy for epidermoid gum cancer.
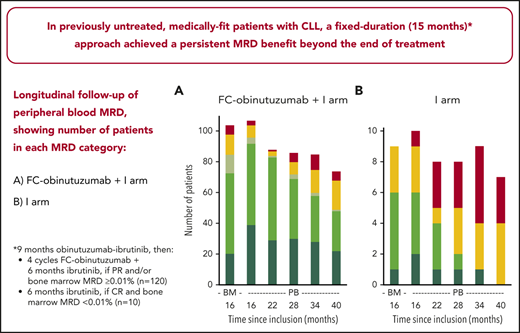
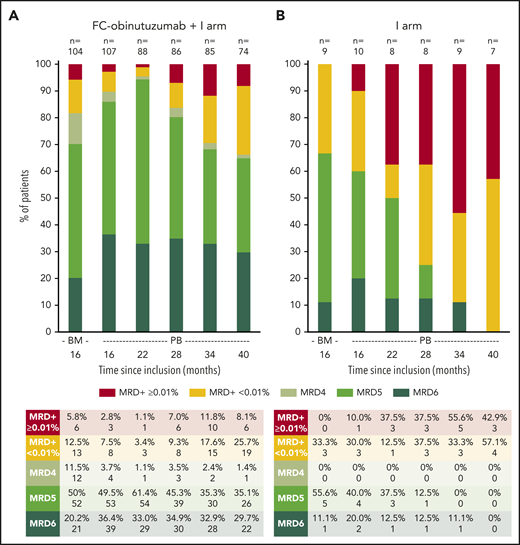
Our study is the first to provide detailed follow-up MRD data beyond end of treatment of a first-line, fixed-duration approach in medically fit patients with CLL. In the ibrutinib arm, the undetectable PB MRD rate decreased rapidly, and at month 40 all patients had either MRD ≥0.01% or low-level positive MRD <0.01% (Figure 2; supplemental Figure 4). In the FC-obinutuzumab plus ibrutinib arm, the undetectable PB MRD rate was 89.7% (96 of 107) at month 16 and increased at month 22. Thereafter, positive PB MRD rates increased; however, over two-thirds of the arm remained with undetectable PB MRD. At month 40, the between-arm difference in classical PB MRD <0.01% status was statistically significant (P = .03). Two patients had CR with BM MRD ≥0.01% at month 16 and so continued ibrutinib: 1 discontinued ibrutinib 22 months later due to toxicity (with no clinical/biological progression, but PB MRD remained ≥0.01%) and 1 discontinued ibrutinib 12 months later upon reaching PB MRD <0.01% (with no clinical/biological progression, but reaching PB MRD ≥0.01% at month 52). In the similar trial evaluating a 12-cycle ibrutinib plus FC-obinutuzumab regimen, no patient had a PB MRD recurrence at median follow-up 18.7 months beyond end of treatment.12 Following 12 cycles of venetoclax-obinutuzumab in patients with coexisting conditions, PB MRD clearance was assessed up to month 30 by allele-specific oligonucleotide polymerase chain reaction, and the undetectable level appeared to be well sustained.11
Longitudinal follow-up of PB MRD, showing proportions of patients in each MRD category, by treatment arm. (A) FC-obinutuzumab plus I arm. (B) I arm. PB MRD was assessed every 6 months from month 16. For each treatment arm, we defined 2 groups with MRD: the first included cases with MRD ≥0.01% and was referred to as positive MRD; and the second included cases with positive MRD <0.01% and was referred to as low-level positive MRD. When the positivity criteria were not met, the MRD status was undetectable and a limit of sensitivity of the test was calculated. Undetectable MRD cases were then subcategorized into 3 groups depending on the limit of sensitivity: MRD4 comprised sensitivity 10−4 to 10−5, MRD5 comprised sensitivity 10−5 to 10−6, and MRD6 comprised sensitivity below 10−6. As we were able to produce high-sensitivity tests, the proportion of cases with MRD4 was low and the proportions with MRD5 and MRD6 were well balanced.
Longitudinal follow-up of PB MRD, showing proportions of patients in each MRD category, by treatment arm. (A) FC-obinutuzumab plus I arm. (B) I arm. PB MRD was assessed every 6 months from month 16. For each treatment arm, we defined 2 groups with MRD: the first included cases with MRD ≥0.01% and was referred to as positive MRD; and the second included cases with positive MRD <0.01% and was referred to as low-level positive MRD. When the positivity criteria were not met, the MRD status was undetectable and a limit of sensitivity of the test was calculated. Undetectable MRD cases were then subcategorized into 3 groups depending on the limit of sensitivity: MRD4 comprised sensitivity 10−4 to 10−5, MRD5 comprised sensitivity 10−5 to 10−6, and MRD6 comprised sensitivity below 10−6. As we were able to produce high-sensitivity tests, the proportion of cases with MRD4 was low and the proportions with MRD5 and MRD6 were well balanced.
Patients in our study with IGHV-mutated status had a higher classical PB MRD <0.01% rate than those with IGHV-unmutated status throughout the follow-up, but the difference was not statistically significant (P = .20 at month 40) (supplemental Figure 5). Today, 6 cycles of FCR typically remains a standard of care in CLL only in medically fit patients with IGHV-mutated status.24 A fixed-duration approach such as ours, with a shorter immunochemotherapy duration, might be an alternative for such patients with good prognostic factors. This is supported by the findings for the similar 12-cycle ibrutinib plus FC-obinutuzumab regimen, in which the trial specifically included patients with IGHV-mutated status.12 A subgroup with IGHV-mutated status from a trial in younger patients found that 6 cycles of ibrutinib-rituximab followed by continuous ibrutinib demonstrated no significant difference in 3-year PFS vs 6 cycles of FCR, and so might be another option for such patients.4,23
Hematologic grade 3/4 adverse events occurring beyond the end of treatment were neutropenia (18% of patients) and lymphopenia (4% of patients); 2 patients had infections (pneumonia grade 4; pulmonary aspergillosis) and 2 had secondary cancers (epidermoid gum cancer; Hodgkin lymphoma). Regarding nonhematologic grade 3/4 events, 3 patients had grade 3 cardiac events (tachycardia, hypertensia, atrial fibrillation). The occurrence of infections and secondary malignancies did not differ from other trials of first-line, fixed-duration approaches containing targeted agents.8,9,11
In summary, our first-line, fixed-duration approach (9 months obinutuzumab-ibrutinib; then 6 months ibrutinib only if CR with BM MRD <0.01%, or ibrutinib 6 months plus 4 cycles FC-obinutuzumab otherwise) achieved a persistent MRD benefit beyond the end of treatment, high 3-year PFS and OS rates, and low long-term toxicity. Further long-term data from other trials evaluating first-line, fixed-duration approaches7-12 are eagerly awaited.
Presented in abstract form (oral presentation) at the 25th European Hematology Association Annual Congress; 11-21 June 2020; Virtual. Abstract S160.
For original data, contact Valerie Rouille (v-rouille@chu-montpellier.fr).
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank the patients who participated in this trial and their families, the trial investigators, and other support staff at the clinical centers. The authors also thank Cath Carsberg (Stockport, United Kingdom) for medical writing assistance in the preparation of this manuscript.
This work was supported by Roche and Janssen, both of which provided funding for this assistance.
Authorship
Contribution: V.R. was head of project, responsible for regulatory authorization and following data collection; P.F. and A.-S.M. performed a literature search; P.F., A.-S.M., L.Y., V. Leblond, O.T., R.L., V. Levy, F.C., and A.D. contributed to the study design; V.R., P.F., and A.-S.M. collected data; F.S., A.-S.M., and P.F. analyzed and interpreted the data; A.-S.M. and P.F. drafted the report; and all authors revised the report for content and approved the final version.
Conflict-of-interest disclosure: R.L. reports personal fees from AbbVie, nonfinancial support from Janssen, and personal fees and nonfinancial support from Alexion. M.L.G.-T. reports personal fees from Alexion Pharma France. M.-S.D. reports personal fees and nonfinancial support from Janssen and AbbVie. K.L. reports grants from Takeda; personal fees from Amgen, Gilead, and Janssen; and grants and personal fees from AbbVie, Novartis, Roche, and Sandoz. G.S. reports grants and personal fees from Roche, and personal fees from AbbVie, Acerta, Amgen, Bristol Myers Squibb, Celgene, Epizyme, Gilead, Janssen, Merck, Morphosys, Novartis, Pfizer, and Servier. O.T. reports personal fees from Takeda, and personal fees and nonfinancial support from AbbVie, Gilead, Janssen, Roche, and Sandoz. A.D. reports personal fees and nonfinancial support from AbbVie, Gilead, Janssen, and Roche. B.P. reports personal fees from Celgene, Janssen, Sebia, and Takeda. V. Leblond reports personal fees and nonfinancial support from AbbVie, Gilead, Janssen, and Roche. P.C. reports personal fees from Novartis and Roche, nonfinancial support from Gilead and Sandoz, and personal fees and nonfinancial support from Takeda. G.C. reports personal fees from Celgene, Gilead, Janssen, and Roche. L.-M.F. reports personal fees from Gilead, Janssen, Roche, and Takeda. L.Y. reports grants from AbbVie, Janssen, Gilead, and Roche. C.D. reports personal fees from Janssen, nonfinancial support from Gilead, and personal fees and nonfinancial support from AbbVie and Roche. F.C. reports grants and personal fees from Sunesis, and personal fees and nonfinancial support from AbbVie, Gilead, Janssen, and Roche. V. Levy reports personal fees from AbbVie, Gilead, Janssen, and Roche. P.F. reports personal fees and nonfinancial support from AbbVie, Gilead, Janssen, and Roche. The remaining authors declare no competing financial interests.
A complete list of the members of participating French Innovative Leukemia Organization (FILO) centers and lead investigators appears in the supplemental Appendix.
Correspondence: Anne-Sophie Michallet, Department of Hematology, Centre Léon Bérard, FR-69008, Lyon, France; e-mail: anne-sophie.michallet@lyon.unicancer.fr.