In this issue of Blood, Daher et al1 present a strategy to improve natural killer (NK) cell effector function that combines chimeric antigen receptor (CAR) engineering and gene editing of a cytokine-related immune checkpoint. Their study provides preclinical data on the mechanistic synergy of these 2 approaches and might have an impact on the future development of NK cell–based cancer immunotherapy.
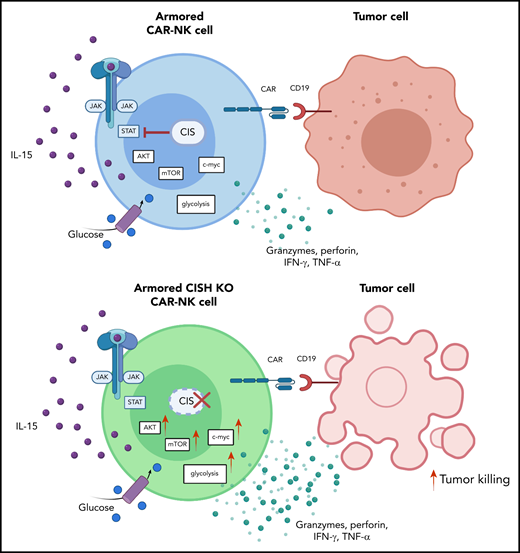
Deletion of CIS in CAR-NK cells enhances IL-15 signaling by removing an immune checkpoint, which leads to increased activation of the AKT/mTOR pathway and, in the presence of tumor cells, enhanced c-Myc activation. These signaling pathways result in greater glycolytic capacity of CAR-NK cells and subsequently increased cytotoxicity against tumor targets. IFN-γ, interferon-γ; JAK, Janus kinase; mTOR, mammalian target of rapamycin; STAT, signal transducer and activator of transcription; TNF-α, tumor necrosis factor-α. See the visual abstract in the article by Daher et al that begins on page 624.
Deletion of CIS in CAR-NK cells enhances IL-15 signaling by removing an immune checkpoint, which leads to increased activation of the AKT/mTOR pathway and, in the presence of tumor cells, enhanced c-Myc activation. These signaling pathways result in greater glycolytic capacity of CAR-NK cells and subsequently increased cytotoxicity against tumor targets. IFN-γ, interferon-γ; JAK, Janus kinase; mTOR, mammalian target of rapamycin; STAT, signal transducer and activator of transcription; TNF-α, tumor necrosis factor-α. See the visual abstract in the article by Daher et al that begins on page 624.
Recent advances in cancer immunotherapy have mainly focused on engineering T cells to express a CAR against specific tumor antigens. Although significant clinical efficacy has been achieved in B-cell malignancies, the application of the existing CAR T-cell products approved by the US Food and Drug Administration still faces logistic and clinical challenges, such as timely collection and expansion of sufficient numbers of autologous gene–modified T cells in heavily pretreated patients. NK cells are attractive alternative candidates for novel approaches in cancer immunotherapy because they mediate potent cytotoxicity against tumor cells and can potentially be used as readily available off-the-shelf products.2
Liu et al have previously demonstrated that cord blood–derived NK cells engineered to secrete interleukin-15 (IL-15) and to express a CD19-targeted CAR exhibit potent antitumor activity and long-term persistence.3 In the Daher et al study, the authors investigate the question of whether the effector function and persistence of these IL-15–secreting, so-called “armored” CD19 CAR-NK cells can be further improved by deleting cytokine-inducible Src homology 2-containing protein (CIS), a key negative regulator of IL-15 signaling.
CIS, which is encoded by the CISH gene, is a member of the suppressor of cytokine signaling (SOCS) family of proteins. CIS is induced by cytokines such as IL-15 and IL-2 and acts as an important intracellular immune checkpoint in NK cells.4 Specifically, it uses a negative feedback loop to suppress signaling of the pleiotropic cytokine IL-15, which is known to drive NK-cell activation, expansion, and persistence.4
By using a series of in vitro and in vivo experiments, the authors provide a detailed characterization of CD19 CAR-NK cells undergoing CRISPR/Cas9-mediated CISH knockout (KO). On a phenotypic level, CISH deletion was shown to be associated with prominent features of activation, proliferation, and cytotoxicity. Interestingly, despite the activation phenotype caused by CISH KO, no evidence of activation-induced cell death was observed after stimulation with tumor targets in vitro. The effects of CISH deletion on the transcriptome included upregulation of genes related to inflammatory and immune responses as well as cytokine signaling. Notably, pathways involved in signaling of interferon-γ, tumor necrosis factor-α, IL-2/STAT5 and IL-6/JAK/STAT3 were enhanced.
Taken together, these results support the hypothesis that targeting CIS in CAR-NK cells removes a critical immune checkpoint. When tested against CD19+ Raji lymphoma cells in vitro, CISH KO CAR-NK cells displayed greater cytotoxicity against their targets. From a metabolic standpoint, the study provides evidence that this gain of effector function can be attributed to enhanced IL-15 signaling secondary to removal of the CIS checkpoint, which leads to activation of the AKT/mTOR pathway and c-Myc pathway (see figure). The authors suggest that endogenously secreted IL-15 by CAR-NK cells has to overcome a lower threshold of activity to trigger the AKT/mTOR pathway, which is known to be involved in proliferation and cytotoxicity.5 In contrast, c-Myc, a mediator of glycolysis,6 was upregulated only in the presence of Raji lymphoma cells, thereby increasing NK-cell metabolic fitness in response to target cells. These results on improved metabolic function contrast with previous findings which indicated that continuous treatment of human NK cells with IL-15 results in functional changes consistent with exhaustion and thus warrants further in-depth analyses.7 When Daher et al used an in vivo Raji lymphoma model in their study, CIS checkpoint disruption combined with CAR engineering improved antitumor activity and the persistence of CAR-NK cells.
Although greater tumor control is desirable, unleashing the effector function of CAR-NK cells raises possible concerns regarding the safety of the proposed strategy. In the given setting, the cells not only ectopically express IL-15, thereby driving inflammatory responses and cell expansion, but in addition, the physiologic negative feedback control of IL-15 signaling is disrupted. With regard to cytokine release syndrome, apparent toxicities such as rapid weight loss or early death were not observed in the present mouse model. However, the NSG mouse model used in the Daher et al study has limitations for assessing cytokine release syndrome, and more detailed investigations will be needed.8 Furthermore, aberrant IL-15 is known to potentially cause NK lymphoma and to initiate malignant transformation in large granular lymphocytes through induction of Myc.9 In the Daher et al study, CISH KO did not lead to uncontrolled growth patterns of NK cells or increased expression of genes related to chromosomal instability or DNA damage. Nevertheless, further investigations on prolonged exposure to IL-15 will be necessary to evaluate potential oncogenic effects of IL-15. With these possible challenges in mind, it is noteworthy that the armored CAR-NK cells used by Daher et al were equipped with an inducible caspase-9 suicide gene as a safety mechanism, which was effective at eliminating CAR-NK cells in vitro and in vivo upon application of a small molecule dimerizer.
In summary, Daher et al propose a strategy for improving NK-cell effector function in the context of IL-15–secreting CD19 CAR-NK cells. Early results of a recent phase 1/2 trial have shown that these CAR-NK cells can induce responses in patients with high-risk CD19+ cancers without major toxic effects.10 The Daher et al study suggests that additional deletion of the immune checkpoint CIS releases the brake on IL-15 signaling, which leads to improved tumor control and CAR-NK–cell persistence, and which is an interesting novel aspect to consider in the development of NK cells for cancer immunotherapy.
Conflict-of-interest disclosure: The author declares no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal