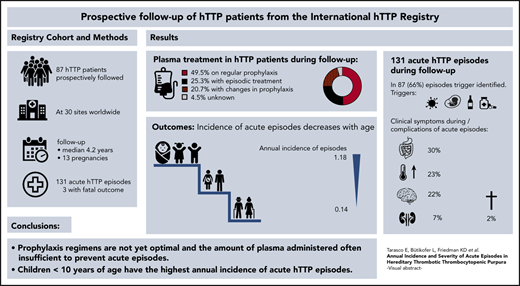
Key Points
Annual incidence of acute episodes of hTTP is highest in children <10 years of age and decreases with increasing age.
Currently applied plasma prophylaxis is often not sufficient to prevent the occurrence of acute episodes of hTTP.
Abstract
Hereditary thrombotic thrombocytopenic purpura (hTTP) is a rare thrombotic microangiopathy characterized by severe congenital ADAMTS13 deficiency and recurring acute episodes causing morbidity and premature death. Information on the annual incidence and severity of acute episodes in patients with hTTP is largely lacking. This study reports prospective data on 87 patients from the Hereditary TTP Registry (clinicaltrials.gov #NCT01257269) for survival, frequency, and severity of acute episodes from enrollment until December 2019. The 87 patients, followed up for a median of 4.2 years (range, 0.01-15 years), had a median age at overt disease onset and at clinical diagnosis of 4.6 years and 18 years (range, 0.0-70 years for both), respectively. Forty-three patients received regular plasma prophylaxis, whereas 22 did not, and treatment changed over time or was unknown in the remaining 22. Forty-three patients experienced 131 acute episodes, of which 91 (69%) occurred in patients receiving regular prophylaxis. This resulted in an annual incidence of acute episodes of 0.36 (95% confidence interval [CI], 0.29-0.44) with regular plasma treatment and of 0.41 (95% CI, 0.30-0.56) without regular plasma treatment. More than one-third of acute episodes (n = 51) were documented in children <10 years of age at enrollment and were often triggered by infections. Their annual incidence of acute episodes was significantly higher than in patients aged >40 years (1.18 [95% CI, 0.88-1.55] vs 0.14 [95% CI, 0.08-0.23]). The prophylactic plasma infusion regimens used were insufficient to prevent acute episodes in many patients. Such regimens are burdensome, and caregivers, patients, and their guardians are reluctant to start regular plasma infusions, from which children particularly would benefit.
Introduction
Hereditary thrombotic thrombocytopenic purpura (hTTP), also known as Upshaw-Schulman syndrome (#274150 Online Mendelian Inheritance in Man), is a rare thrombotic microangiopathy characterized by severe congenital ADAMTS13 deficiency and smoldering disease. It also involves acute disease episodes with thrombocytopenia, hemolytic anemia, and microvascular thrombosis leading to end-organ damage (mainly affecting brain and kidneys).1-3 ADAMTS13 is essential for cleaving hemostatically very active, ultra-large von Willebrand factor (vWF) multimers into less sticky, regular-sized multimers to prevent spontaneous platelet adhesion and eventually the formation of vWF- and platelet-rich thrombi occluding the microcirculation. Inheritance of hTTP is autosomal recessive, and today >150 causative ADAMTS13 mutations have been reported.4-8 hTTP has an estimated prevalence of 0.5 to 2 cases per 1 million,3 although the prevalence may be considerably higher in certain areas.9
Even with identical ADAMTS13 genotypes, patients with hTTP manifest with variable disease onset and exhibit heterogeneous clinical courses.7-10 Although some patients seemingly remain without disease episodes far into adulthood, others require exchange blood transfusion in the first days of life and/or experience already numerous acute TTP episodes in childhood.7-12 Although hTTP can become overt at any time during a patient’s life,3,7,8 early childhood and pregnancy are periods of high risk of manifestation.11-17
Acute TTP episodes in hTTP occur spontaneously or are triggered by factors associated with increased vWF plasma levels, endothelial cell activation, or increased shear stress such as a patent ductus arteriosus,18 infections,5,8,12,17 pregnancy,5,8,13-16 alcohol,5,8 and certain drugs (desmopressin).5,19 The high number of patients with hTTP having experienced arterial thrombotic events already at a relatively young age indicates the significance of acute episodes.20 At enrollment into the international Hereditary TTP Registry, 25 (21%) of 120 patients reported to have had a stroke, 5 patients a transient ischemic attack, and another 5 patients a myocardial infarction.8 Preexisting arterial thrombotic events were present in >50% of patients with hTTP enrolled at age 40 to 50 years and older. Although no myocardial infarction was observed in the UK hTTP cohort, 14 (19%) of 73 patients had experienced a stroke.7 Regular plasma infusions, usually administered in fortnightly intervals, are effective in preventing acute TTP episodes in individual patients over years.5,7,8,21,22 Prophylactic plasma therapy is associated with regular visits at treatment centers, is time-consuming, and allergic reactions to plasma products are frequent; the decision to initiate such a regimen is not easily taken. Still, 71% of patients with hTTP at enrollment into the International Hereditary TTP Registry were on regular plasma prophylaxis,8 and a similar number (69%) was reported for the UK cohort.7
At present, no data are available regarding the annual incidence of acute episodes in hTTP patients or regarding the frequency and severity of episodes in patients on regular plasma prophylaxis. There is also no information on the long-term advantage of this burdensome treatment.
We addressed these questions by investigating the occurrence, the severity of acute disease episodes, and survival as well as treatment over time in hTTP patients in the international Hereditary TTP Registry. The analysis was based on prospective follow-up data from patients’ enrollment until their last scheduled visit by the end of 2019.
Materials and methods
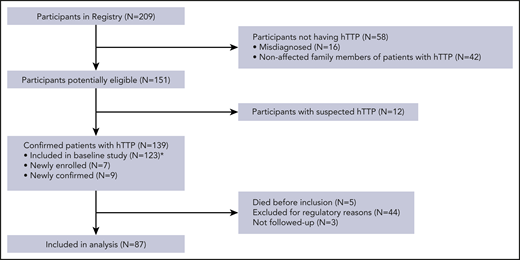
The Hereditary TTP Registry (www.ttpregistry.net; clinicaltrials.gov #NCT01257269) is an ambidirectional cohort study for patients diagnosed with hTTP and their family members.8,23 The Kantonale Ethik-Kommission Bern, as the lead ethics committee (EC), approved the study in September 2006 (study number 031/06). Before enrollment of patients, each participating site obtained approval from its local or national institutional review board or EC. Due to regulatory obligations, renewal of EC approval at all Japanese sites is underway, and all Japanese registry participants have to undergo re-consenting, which is pending in 44. These 44 patients were consequently not included in this follow-up analysis.
At the end of 2012, paper case report forms were replaced by web-based, electronic case report forms with an associated database (webspirit, 2mt software, Ulm, Germany). This enables automatic e-mail reminders of upcoming follow-up visits and allows the sites to enter new data directly after personalized login. Central project coordination, including data monitoring and cleaning, is conducted at Bern University Hospital.
Patients and data collection
For this first prospective study, confirmed hTTP patients8 having an enrollment date before August 1, 2018, and at least one follow-up visit, or a study-end visit by the end of 2019, were considered eligible. A follow-up visit was defined as any visit, face-to-face or by telephone call, that happened after enrollment. Per study protocol, prospective follow-up visits were to be performed annually and at occurrence of any acute episode. Not all patients were followed up annually, although adherence improved during recent years. The mean and maximal gap between follow-up visits was 0.95 year (range, 0.01-5.6 years) and 1.6 years (range, 0.01-8.2 years), respectively, with the majority of patients (n = 54) having a maximal gap of 1 to 2 years between follow-up visits (supplemental Table 1, available on the Blood Web site).
We analyzed the following data: course of hTTP since enrollment, incidence, and severity of acute episodes; survival; treatment, including regular plasma therapy and changes thereof; intake of antihypertensive, antiplatelet, and/or anticoagulant drugs; treatment side effects; and pregnancies.
To calculate a patient’s annual volume of plasma received for prophylaxis, we set the volume of unit of solvent detergent plasma as 200 mL (standardized volume of Octaplas [Octapharma, Lachen, Switzerland ], the most widely used solvent detergent plasma in our cohort), and of fresh frozen plasma as 250 mL (the volume of 1 unit of fresh frozen plasma typically ranges from 200-300 mL). In the absence of information from the center caring for the respective patient, we assumed that the number of plasma units administered per session, the treatment interval, and body weight were stable between 2 visits.
Specification and severity of an acute TTP episode
An acute TTP episode was defined as a typical TTP episode,24 or an acute cerebrovascular or cardiovascular or other ischemic event, or a renal illness for which the patient required and/or received medical care in addition to the patient’s regular prophylactic treatment (eg, with plasma infusion). In addition, a maternal pregnancy complication resulting in pregnancy termination (abortion or delivery) after 10 weeks and before 34 weeks of gestation was considered an acute episode (Table 1). Four categories were defined to grade the severity of the acute TTP episode, with category 1 characterized by mild clinical manifestations; categories 2 and 3 by moderate to severe clinical symptoms and signs; and category 4 by fatal outcome. Episodes were scored by the registry project manager (E.T.) and at least one registry steering committee member (B.L. and/or J.A.K.H.) independently. In case of discordant scores, the hTTP episodes in question were discussed again with the treating physician and then the entire registry team at Bern University Hospital until a consensus was reached.
Definition of acute hTTP episodes and grading system to assess their severity
| Acute hTTP episodes | A typical TTP episode,24 or an acute cerebrovascular or cardiovascular or other ischemic event; or a renal illness for which the patient requires and/or receives medical care in addition to regular prophylactic treatment (eg, with plasma infusions) |
| In addition, maternal pregnancy complications resulting in a premature termination of pregnancy by abortion or delivery after 10 wk and before 34 wk of gestation | |
| If laboratory assays are performed, they typically show: | |
| Thrombocytopenia | |
| Presence of schistocytes on peripheral blood smear | |
| Decreased hemoglobin or reduced hematocrit | |
| Decreased haptoglobin | |
| Increased lactate dehydrogenase | |
| Increased (indirect) bilirubin | |
| Increased reticulocyte count | |
| Mild episode = severity score 1 | Typical signs and symptoms: |
| Petechiae, ecchymoses/bruises, small hematomas | |
| Cephalgia, vertigo, nausea | |
| Fatigue, drowsiness, weakness, | |
| (Sub)-febrile temperatures, shivering | |
| Gastrointestinal symptoms (eg vomiting, abdominal discomfort, diarrhea) | |
| Microscopic hematuria or macroscopic hematuria | |
| Hemolytic jaundice | |
| Tachycardia, moderate dyspnea | |
| Moderate episode = severity score 2 | Typical signs and symptoms: |
| Transient ischemic attack | |
| Angina pectoris | |
| Cardiac arrhythmia | |
| Acute renal failure, not requiring renal replacement | |
| Pregnancy-associated TTP event with abortion <10th week of gestation | |
| Severe episode = severity score 3 | Typical signs and symptoms: |
| Ischemic stroke | |
| Coma, seizure, or impaired consciousness requiring airway-protective measures and ventilation | |
| Acute myocardial infarction | |
| Acute renal failure requiring renal replacement therapy | |
| Multiorgan failure | |
| Pregnancy with abortion ≥10th wk of gestation, or a premature delivery <34th wk of gestation (maternal indication) | |
| Fatal episode = severity score 4 | Fatal outcome of a typical TTP episode and/or death from a cerebrovascular or cardiovascular event, or death during pregnancy |
| Acute hTTP episodes | A typical TTP episode,24 or an acute cerebrovascular or cardiovascular or other ischemic event; or a renal illness for which the patient requires and/or receives medical care in addition to regular prophylactic treatment (eg, with plasma infusions) |
| In addition, maternal pregnancy complications resulting in a premature termination of pregnancy by abortion or delivery after 10 wk and before 34 wk of gestation | |
| If laboratory assays are performed, they typically show: | |
| Thrombocytopenia | |
| Presence of schistocytes on peripheral blood smear | |
| Decreased hemoglobin or reduced hematocrit | |
| Decreased haptoglobin | |
| Increased lactate dehydrogenase | |
| Increased (indirect) bilirubin | |
| Increased reticulocyte count | |
| Mild episode = severity score 1 | Typical signs and symptoms: |
| Petechiae, ecchymoses/bruises, small hematomas | |
| Cephalgia, vertigo, nausea | |
| Fatigue, drowsiness, weakness, | |
| (Sub)-febrile temperatures, shivering | |
| Gastrointestinal symptoms (eg vomiting, abdominal discomfort, diarrhea) | |
| Microscopic hematuria or macroscopic hematuria | |
| Hemolytic jaundice | |
| Tachycardia, moderate dyspnea | |
| Moderate episode = severity score 2 | Typical signs and symptoms: |
| Transient ischemic attack | |
| Angina pectoris | |
| Cardiac arrhythmia | |
| Acute renal failure, not requiring renal replacement | |
| Pregnancy-associated TTP event with abortion <10th week of gestation | |
| Severe episode = severity score 3 | Typical signs and symptoms: |
| Ischemic stroke | |
| Coma, seizure, or impaired consciousness requiring airway-protective measures and ventilation | |
| Acute myocardial infarction | |
| Acute renal failure requiring renal replacement therapy | |
| Multiorgan failure | |
| Pregnancy with abortion ≥10th wk of gestation, or a premature delivery <34th wk of gestation (maternal indication) | |
| Fatal episode = severity score 4 | Fatal outcome of a typical TTP episode and/or death from a cerebrovascular or cardiovascular event, or death during pregnancy |
ADAMTS13 parameters
In 70 of 87 patients, ADAMTS13 activity was determined at Bern University Hospital (University of Bern, Bern, Switzerland) by using the modified FRETS-VWF73 assay.25,26 In the remaining patients, ADAMTS13 activity was assessed in other laboratories using various assay methods (n = 15); it was not reported for 2 patients.
To assess a patient’s baseline ADAMTS13 activity reflecting endogenous ADAMTS13 production, only blood samples withdrawn at least 14 days after the last administration of plasma-containing blood products were considered.
Functional ADAMTS13 inhibitors (positive results >0.4 Bethesda units/mL), anti-ADAMTS13 antibodies by enzyme-linked immunosorbent assay (TECHNOZYM® ADAMTS13 INH ELISA, Technoclone GmbH, Vienna, Austria; positive result >15 arbitrary units/mL), and genetic analysis of ADAMTS13 were performed at Bern University Hospital, as previously described.8,9
Statistical analysis
Categorical variables are reported as numbers and percentages, and were compared between groups with Fisher’s exact test. Continuous data are reported as median and range (and 25th and 75th percentiles for certain variables of interest), and compared by using Wilcoxon rank sum tests.
Incidence rates are presented with exact Poisson 95% confidence intervals (CIs). Because it was not possible to follow up all patients annually, we assessed the influence of large gaps between follow-up visits (that could lead to an underreporting of episodes) on the annual incidence rate estimates by a series of sensitivity analyses. We assumed a patient was lost to follow-up in case of gaps between visits of >2, 3, and 5 years, respectively (supplemental Table 2). Because the trend of results indicated that larger gaps were not affecting our results, we included all 87 confirmed hTTP patients in the current analysis irrespective of their differences in follow-up years.
The effect of age at enrollment, sex, use of prophylaxis, and follow-up time on the incidence of TTP episodes was analyzed by using mixed effects Poisson regression with robust standard errors based on the Huber-White sandwich estimator.
Stata version 15 (StataCorp, College Station, TX) was used for all statistical analyses.
Results
Patient overview: clinical and biochemical characteristics
At the end of December 2019, the Hereditary TTP Registry had 209 participants, of whom 139 were confirmed hTTP patients (Figure 1). Five confirmed hTTP patients had died before inclusion into the registry, 3 patients were lost to follow-up, and 44 Japanese patients were temporarily suspended because of regulatory reasons. Hence, 87 patients with hTTP (46 male patients and 41 female patients) contributed to this follow-up study. Their median follow-up time was 4.2 years (range, 0.01-15 years), and they had a median of 3 follow-up visits (range, 1-10 visits).
Study flowchart. Participants in the International Hereditary TTP Registry at the end of 2019 and confirmed hTTP patients followed up prospectively. *Patients described in van Dorland et al.8
Study flowchart. Participants in the International Hereditary TTP Registry at the end of 2019 and confirmed hTTP patients followed up prospectively. *Patients described in van Dorland et al.8
Most patients were White (n = 75 [86%]), followed by Asian (n = 6 [6.9%]), Hispanic (n = 3 [3.4%]), and other (n = 3 [3.4%]). Nine patients had consanguineous parents, and 13 had a family member who was also affected by hTTP (one parent–child relation; the others were siblings). Further demographic and clinical features, as well as treatments, are listed in Table 2.
Baseline demographic and clinical characteristics of 87 hTTP patients, including 43 with and 44 without acute episodes during follow-up
| Characteristic . | All patients . | No TTP episodes during follow-up . | Acute TTP episodes during follow-up . | P* . | |||
|---|---|---|---|---|---|---|---|
| N . | n (%) or median [min, lq, uq, max] . | N . | n (%) or median [min, lq, uq, max] . | N . | n (%) or median [min, lq, uq, max] . | ||
| Sex | 87 | 44 | 43 | .13 | |||
| Male | 46 (53%) | 27 (61%) | 19 (44%) | ||||
| Female | 41 (47%) | 17 (39%) | 24 (56%) | ||||
| Consanguinity of parents | 67 | 9 (13%) | 32 | 6 (19%) | 35 | 3 (8.6%) | .29 |
| Family member is confirmed patient | 87 | 13 (15%) | 44 | 5 (11%) | 43 | 8 (19%) | .38 |
| Age, y, at | |||||||
| Overt disease onset | 77 | 4.6 [0.0, 0.0, 19, 70] | 35 | 4.7 [0.0, 0.0, 20, 70] | 42 | 3.6 [0.0, 0.0, 19, 68] | .80 |
| Clinical diagnosis | 85 | 18 [0.0, 4.2, 29, 70] | 42 | 20 [0.0, 9.4, 33, 70] | 43 | 15 [0.0, 3.5, 28, 68] | .12 |
| Enrollment | 87 | 26 [1.2, 16, 40, 75] | 44 | 27 [1.7, 18, 44, 75] | 43 | 26 [1.2, 14, 37, 69] | .33 |
| The last follow-up | 87 | 32 [2.1, 21, 46, 79] | 44 | 31 [5.2, 22, 48, 79] | 43 | 32 [2.1, 21, 42, 73] | .51 |
| Treatment | |||||||
| Prophylactic plasma infusions | 83 | 47 (57%) | 40 | 24 (60%) | 43 | 23 (53%) | .66 |
| Antiaggregation/anticoagulation | 79 | 21 (27%) | 37 | 12 (32%) | 42 | 9 (21%) | .31 |
| Antihypertensive medication | 79 | 25 (32%) | 37 | 14 (38%) | 42 | 11 (26%) | .33 |
| Comorbidities at enrollment | |||||||
| Arterial thrombotic disease | 83 | 30 (36%) | 41 | 19 (46%) | 42 | 11 (26%) | .07 |
| Myocardial infarction | 83 | 4 (4.8%) | 41 | 2 (4.9%) | 42 | 2 (4.8%) | 1.00 |
| Transient ischemic attack | 83 | 13 (16%) | 41 | 7 (17%) | 42 | 6 (14%) | .77 |
| Stroke | 83 | 22 (27%) | 41 | 15 (37%) | 42 | 7 (17%) | .049 |
| Other | 83 | 4 (4.8%) | 41 | 3 (7.3%) | 42 | 1 (2.4%) | .36 |
| Neurologic disorders | 83 | 18 (22%) | 41 | 13 (32%) | 42 | 5 (12%) | .035 |
| Epileptic seizure | 83 | 6 (7.2%) | 41 | 6 (15%) | 42 | 0 (0%) | .012 |
| Headache | 83 | 3 (3.6%) | 41 | 2 (4.9%) | 42 | 1 (2.4%) | .62 |
| Other neurologic disease | 83 | 12 (14%) | 41 | 8 (20%) | 42 | 4 (10%) | .23 |
| Venous thrombotic disease | 83 | 3 (3.6%) | 41 | 3 (7.3%) | 42 | 0 (0%) | .12 |
| Renal insufficiency | 83 | 26 (31%) | 41 | 11 (27%) | 42 | 15 (36%) | .48 |
| Pathologic renal parameters | 83 | 17 (20%) | 41 | 7 (17%) | 42 | 10 (24%) | .59 |
| On dialysis | 83 | 8 (10%) | 41 | 2 (4.9%) | 42 | 6 (14%) | .26 |
| Kidney transplantation | 83 | 2 (2.4%) | 41 | 0 (0%) | 42 | 2 (4.8%) | .49 |
| Neonatal hyperbilirubinemia | 83 | 36 (43%) | 41 | 16 (39%) | 42 | 20 (48%) | .51 |
| Characteristic . | All patients . | No TTP episodes during follow-up . | Acute TTP episodes during follow-up . | P* . | |||
|---|---|---|---|---|---|---|---|
| N . | n (%) or median [min, lq, uq, max] . | N . | n (%) or median [min, lq, uq, max] . | N . | n (%) or median [min, lq, uq, max] . | ||
| Sex | 87 | 44 | 43 | .13 | |||
| Male | 46 (53%) | 27 (61%) | 19 (44%) | ||||
| Female | 41 (47%) | 17 (39%) | 24 (56%) | ||||
| Consanguinity of parents | 67 | 9 (13%) | 32 | 6 (19%) | 35 | 3 (8.6%) | .29 |
| Family member is confirmed patient | 87 | 13 (15%) | 44 | 5 (11%) | 43 | 8 (19%) | .38 |
| Age, y, at | |||||||
| Overt disease onset | 77 | 4.6 [0.0, 0.0, 19, 70] | 35 | 4.7 [0.0, 0.0, 20, 70] | 42 | 3.6 [0.0, 0.0, 19, 68] | .80 |
| Clinical diagnosis | 85 | 18 [0.0, 4.2, 29, 70] | 42 | 20 [0.0, 9.4, 33, 70] | 43 | 15 [0.0, 3.5, 28, 68] | .12 |
| Enrollment | 87 | 26 [1.2, 16, 40, 75] | 44 | 27 [1.7, 18, 44, 75] | 43 | 26 [1.2, 14, 37, 69] | .33 |
| The last follow-up | 87 | 32 [2.1, 21, 46, 79] | 44 | 31 [5.2, 22, 48, 79] | 43 | 32 [2.1, 21, 42, 73] | .51 |
| Treatment | |||||||
| Prophylactic plasma infusions | 83 | 47 (57%) | 40 | 24 (60%) | 43 | 23 (53%) | .66 |
| Antiaggregation/anticoagulation | 79 | 21 (27%) | 37 | 12 (32%) | 42 | 9 (21%) | .31 |
| Antihypertensive medication | 79 | 25 (32%) | 37 | 14 (38%) | 42 | 11 (26%) | .33 |
| Comorbidities at enrollment | |||||||
| Arterial thrombotic disease | 83 | 30 (36%) | 41 | 19 (46%) | 42 | 11 (26%) | .07 |
| Myocardial infarction | 83 | 4 (4.8%) | 41 | 2 (4.9%) | 42 | 2 (4.8%) | 1.00 |
| Transient ischemic attack | 83 | 13 (16%) | 41 | 7 (17%) | 42 | 6 (14%) | .77 |
| Stroke | 83 | 22 (27%) | 41 | 15 (37%) | 42 | 7 (17%) | .049 |
| Other | 83 | 4 (4.8%) | 41 | 3 (7.3%) | 42 | 1 (2.4%) | .36 |
| Neurologic disorders | 83 | 18 (22%) | 41 | 13 (32%) | 42 | 5 (12%) | .035 |
| Epileptic seizure | 83 | 6 (7.2%) | 41 | 6 (15%) | 42 | 0 (0%) | .012 |
| Headache | 83 | 3 (3.6%) | 41 | 2 (4.9%) | 42 | 1 (2.4%) | .62 |
| Other neurologic disease | 83 | 12 (14%) | 41 | 8 (20%) | 42 | 4 (10%) | .23 |
| Venous thrombotic disease | 83 | 3 (3.6%) | 41 | 3 (7.3%) | 42 | 0 (0%) | .12 |
| Renal insufficiency | 83 | 26 (31%) | 41 | 11 (27%) | 42 | 15 (36%) | .48 |
| Pathologic renal parameters | 83 | 17 (20%) | 41 | 7 (17%) | 42 | 10 (24%) | .59 |
| On dialysis | 83 | 8 (10%) | 41 | 2 (4.9%) | 42 | 6 (14%) | .26 |
| Kidney transplantation | 83 | 2 (2.4%) | 41 | 0 (0%) | 42 | 2 (4.8%) | .49 |
| Neonatal hyperbilirubinemia | 83 | 36 (43%) | 41 | 16 (39%) | 42 | 20 (48%) | .51 |
lq, lower quartile; max, maximum; min, minimum; n, number of patients in each category; N, number of patients with information available; uq, upper quartile.
Comparison of groups with and without acute episodes during follow-up.
The median age at overt disease onset of the 87 patients with hTTP was 4.6 years, and at clinical diagnosis it was 18 years (range for both, 0.0-70 years); at enrollment into the registry and at last follow-up, it was 26 years (range, 1.2-75 years) and 32 years (range, 2.1-79 years), respectively (Table 2). Patients who experienced acute episodes during prospective follow-up had a similar age at disease onset but a slightly, although statistically not significant, younger age at clinical diagnosis than those without events (15 [range, 0.0-68] years vs 20 [range, 0.0-70] years; P = .12). Overall, 36 patients had a history of neonatal hyperbilirubinemia, 22 of ischemic strokes, and 13 of transient ischemic attacks; 8 patients were on dialysis, and 2 had received a kidney transplant. Of note, preexisting conditions at enrollment (strokes and epileptic seizures) were more common in patients without acute episodes during follow-up (Table 2).
Forty-three patients were homozygous, and 44 were compound heterozygous ADAMTS13 mutation carriers. ADAMTS13 activity was ≤10% in all 87 patients with hTTP; in 70 of them, ADAMTS13 activity was assessed in the laboratory in Bern University Hospital. Even though ADAMTS13 functional inhibitors were negative in all 77 patients tested, 7 (10.3%) of 68 patients tested had a positive anti-ADAMTS13 antibody enzyme-linked immunosorbent assay result, with antibody titers between 16.2 and 33.8 AU/mL (data not shown).
Occurrence of acute episodes during follow-up overall and in relation to age, sex, and plasma prophylaxis
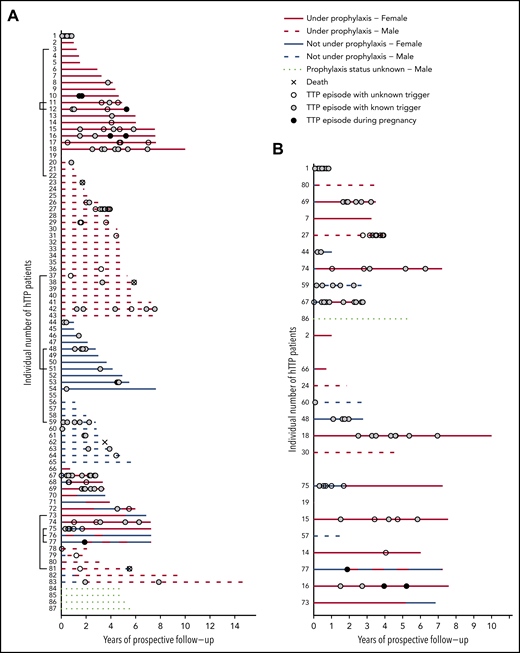
Figure 2A presents the complete overview of prospective follow-up from enrollment until the patients’ last scheduled visit until the end of December 2019 in the 87 patients with hTTP. Patients were sorted according to their sex, prospective observation time, prophylactic treatment, and changes thereof. In addition, we show the occurrence of acute TTP episodes. Forty-three (49.4% of the whole cohort) patients were always on regular prophylactic treatment, whereas 22 (25%) had no prophylactic plasma treatment during follow-up. Eighteen patients received intermittent prophylaxis, including 2 women who were on regular plasma infusions only during pregnancies (patients #76 and #77). Details about their prophylactic regimen were not available for 4 patients (patients #84-#87).
Individual disease courses in 87 patients with hTTP (46 male patients and 41 female patients) after enrollment until last visit up to December 2019. (A) Follow-up (in years) is given on the x-axis; individual patients are listed on the y-axis. Brackets (to the left of the patients’ numbers) link patients with affected family members participating in the registry. Patients are sorted according to sex and use of prophylaxis. (B) Extract of the whole cohort, depicting patients aged <18 years at enrollment (n = 25), sorted according to age, starting with the youngest child. Patients are divided (from uppermost to lowest on the ordinate) in groups of 0 to <6 years of age (n = 11), 6 to <12 years of age (n = 6), and 12 to <18 years of age (n = 8).
Individual disease courses in 87 patients with hTTP (46 male patients and 41 female patients) after enrollment until last visit up to December 2019. (A) Follow-up (in years) is given on the x-axis; individual patients are listed on the y-axis. Brackets (to the left of the patients’ numbers) link patients with affected family members participating in the registry. Patients are sorted according to sex and use of prophylaxis. (B) Extract of the whole cohort, depicting patients aged <18 years at enrollment (n = 25), sorted according to age, starting with the youngest child. Patients are divided (from uppermost to lowest on the ordinate) in groups of 0 to <6 years of age (n = 11), 6 to <12 years of age (n = 6), and 12 to <18 years of age (n = 8).
In Figure 2B, the 25 patients aged <18 years (children) at enrollment are shown, sorted according to their age at enrollment. Although 10 children had no acute episode during prospective follow-up, the other 15 experienced 75 (57.3%) of the 131 documented acute episodes. Patients #1, #15, #16, #18, #27, #67, #69, and #74 experienced multiple acute episodes despite undergoing regular plasma prophylaxis, indicating that the chosen and/or supportable regimens were insufficient.
The 87 confirmed hTTP patients had 371 person-years of prospective follow-up. During follow-up, 44 patients (17 female patients) had no acute TTP episode, whereas the other 43 patients (24 female patients) experienced 131 acute episodes altogether (Table 3). This resulted in an overall annual incidence rate of acute episodes of 0.35 per person-year (95% CI, 0.29-0.42). The annual incidence rates of patients on or not on regular plasma prophylaxis were similar (Figure 3B-E; Table 3).
Incidence of documented acute episodes during follow-up according to sex, age at enrollment, and prophylactic plasma treatment
| Variable . | Patients with follow-up . | Patients with any episode . | Total prospective episodes . | Total person-years . | Annual incidence rate (95% CI) . |
|---|---|---|---|---|---|
| Overall | 87 | 43 | 131 | 371 | 0.35 (0.29-0.42) |
| Male sex | 46 | 19 | 43 | 194 | 0.22 (0.16-0.30) |
| Female sex | 41 | 24 | 88 | 177 | 0.50 (0.40-0.61) |
| Age at enrollment, y | |||||
| <10 | 15 | 9 | 51 | 43 | 1.18 (0.88-1.55) |
| 10-20 | 16 | 8 | 27 | 78 | 0.35 (0.23-0.50) |
| 20-30 | 18 | 8 | 21 | 75 | 0.28 (0.17-0.43) |
| 30-40 | 15 | 9 | 17 | 65 | 0.26 (0.15-0.42) |
| >40 | 23 | 9 | 15 | 110 | 0.14 (0.08-0.23) |
| Prophylaxis* | |||||
| Yes | 61 | 31 | 91 | 254 | 0.36 (0.29-0.44) |
| No | 40 | 17 | 40 | 97 | 0.41 (0.30-0.56) |
| Variable . | Patients with follow-up . | Patients with any episode . | Total prospective episodes . | Total person-years . | Annual incidence rate (95% CI) . |
|---|---|---|---|---|---|
| Overall | 87 | 43 | 131 | 371 | 0.35 (0.29-0.42) |
| Male sex | 46 | 19 | 43 | 194 | 0.22 (0.16-0.30) |
| Female sex | 41 | 24 | 88 | 177 | 0.50 (0.40-0.61) |
| Age at enrollment, y | |||||
| <10 | 15 | 9 | 51 | 43 | 1.18 (0.88-1.55) |
| 10-20 | 16 | 8 | 27 | 78 | 0.35 (0.23-0.50) |
| 20-30 | 18 | 8 | 21 | 75 | 0.28 (0.17-0.43) |
| 30-40 | 15 | 9 | 17 | 65 | 0.26 (0.15-0.42) |
| >40 | 23 | 9 | 15 | 110 | 0.14 (0.08-0.23) |
| Prophylaxis* | |||||
| Yes | 61 | 31 | 91 | 254 | 0.36 (0.29-0.44) |
| No | 40 | 17 | 40 | 97 | 0.41 (0.30-0.56) |
For 4 patients with 21 person-years and 0 episodes, data on prophylaxis were not available; 18 patients had follow-up time with and without prophylaxis (5 with episodes).
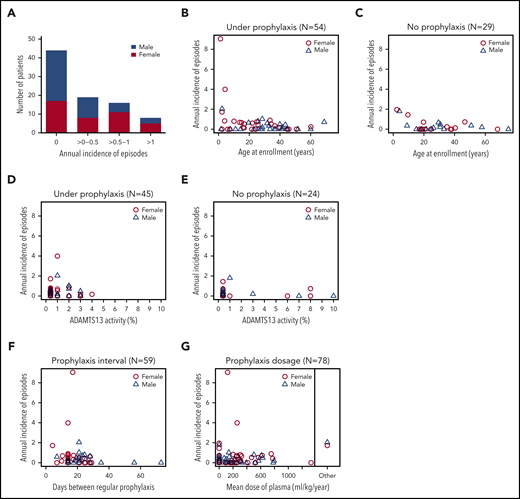
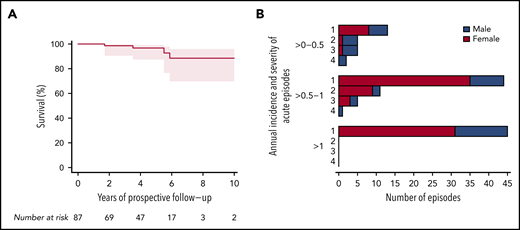
Annual incidence of acute TTP episodes in 87 confirmed hTTP patients based on prospective follow-up until their last visit up to the end of 2019. (A) Patients grouped according to their annual incidence of acute TTP episodes. Although 44 patients with hTTP (50.6%; 27 male subjects, 17 female subjects) experienced no acute episode during follow-up, an annual incidence of >0 to 0.5 was documented in 19 (21.8%; 8 female subjects), of >0.5 to 1 in 16 (18.4%; 11 female subjects), and of >1 in 8 (9.2%; 5 female subjects) patients with hTTP. Annual incidence of acute TTP episodes in individual patients according to age at enrollment: patients were receiving plasma prophylaxis (n = 54) (B) or had no plasma prophylaxis (n = 29) (C) at the last follow-up visit. For 4 patients, information on plasma prophylaxis was missing. Annual incidence of acute TTP episodes in relation to ADAMTS13 activity in 69 patients with hTTP having undergone ADAMTS13 activity measurements by using the modified FRETS-VWF73 assay.25,26 To assess baseline (endogenous) ADAMTS13 activity, blood samples were withdrawn at least 14 days after the last administration of plasma-containing blood products. Patients were receiving plasma prophylaxis (D) or had no plasma prophylaxis (E) at the last follow-up. Annual incidence of acute TTP episodes in relation to interval of regular plasma prophylaxis (F) and to the mean dose of plasma administered per kilogram body weight and year in 78 patients with hTTP with complete data sets for this analysis (G). The 2 patients regularly treated with a plasma-derived factor VIII concentrate are shown separately.
Annual incidence of acute TTP episodes in 87 confirmed hTTP patients based on prospective follow-up until their last visit up to the end of 2019. (A) Patients grouped according to their annual incidence of acute TTP episodes. Although 44 patients with hTTP (50.6%; 27 male subjects, 17 female subjects) experienced no acute episode during follow-up, an annual incidence of >0 to 0.5 was documented in 19 (21.8%; 8 female subjects), of >0.5 to 1 in 16 (18.4%; 11 female subjects), and of >1 in 8 (9.2%; 5 female subjects) patients with hTTP. Annual incidence of acute TTP episodes in individual patients according to age at enrollment: patients were receiving plasma prophylaxis (n = 54) (B) or had no plasma prophylaxis (n = 29) (C) at the last follow-up visit. For 4 patients, information on plasma prophylaxis was missing. Annual incidence of acute TTP episodes in relation to ADAMTS13 activity in 69 patients with hTTP having undergone ADAMTS13 activity measurements by using the modified FRETS-VWF73 assay.25,26 To assess baseline (endogenous) ADAMTS13 activity, blood samples were withdrawn at least 14 days after the last administration of plasma-containing blood products. Patients were receiving plasma prophylaxis (D) or had no plasma prophylaxis (E) at the last follow-up. Annual incidence of acute TTP episodes in relation to interval of regular plasma prophylaxis (F) and to the mean dose of plasma administered per kilogram body weight and year in 78 patients with hTTP with complete data sets for this analysis (G). The 2 patients regularly treated with a plasma-derived factor VIII concentrate are shown separately.
Current recommendations foresee 10 to 15 mL of plasma/kg body weight every 2 to 3 weeks,3,22,27 an interval met in two-thirds of patients on prophylaxis in our cohort (Figure 3F). The 4 patients with the highest annual incidence rates of acute TTP episodes received plasma every 7 days, 14 days (two cases), and 21 days, respectively. The mean annual plasma volume normalized to body weight was ≤400 mL/kg per year (or ≤15 mL/kg every 2 weeks) in 79% (60 of 76) of patients with available information (Figure 3G). The patients with annual plasma volumes >400 mL/kg had annual incidence rates of acute episodes ≤1. During the final weeks of pregnancy, female patients with hTTP received the highest doses of plasma, which amounted to 30 to 40 mL/kg per week (which projects to ∼1500-2000 mL/kg per year).
Patients aged <10 years at enrollment had the highest incidence rate (1.18 [95% CI, 0.88-1.55]), which decreased with each decade and was 0.14 [95% CI, 0.08-0.23] in patients aged >40 years (Table 3). Seven of the eight patients with annual incidence rates >1 were children between 2 and 13 years of age (patients #1, #27, #44, #48, #59, #67, and #69; one adult patient, #20) (Figures 2, 3B-C). Our data show that current prophylactic plasma therapy is often insufficient, particularly in children in whom acute TTP episodes were frequent.
Multivariable analysis considering the effects of age at enrollment, sex, use of prophylaxis, and total follow-up time on the annual incidence of TTP episodes confirmed age and further showed that time of follow-up were the main drivers of the annual incidence rates (Table 4). Increasing age reduced the incidence of TTP episodes by a factor of 0.71 (95% CI, 0.56-0.90) per decade. The apparently higher annual incidence in female patients (Figure 3A) was attributable to their overall younger age (21 years [range 1.2-68] vs 31 years [range 1.7-75] in male subjects; P = .028).
Effect of age at enrollment, sex, use of prophylaxis, and total follow-up time on the incidence of documented TTP episodes during follow-up
| Variable . | Incidence rate ratio (95% CI) . | P . |
|---|---|---|
| Age at enrollment (per decade) | 0.71 (0.56-0.90) | .005 |
| Sex | ||
| Male | 1 (Reference) | |
| Female | 1.49 (0.80-2.77) | .21 |
| Under prophylaxis | ||
| No | 1 (Reference) | |
| Yes | 0.72 (0.39-1.35) | .31 |
| Follow-up time (per year) | 0.89 (0.79-1.01) | .07 |
| Variable . | Incidence rate ratio (95% CI) . | P . |
|---|---|---|
| Age at enrollment (per decade) | 0.71 (0.56-0.90) | .005 |
| Sex | ||
| Male | 1 (Reference) | |
| Female | 1.49 (0.80-2.77) | .21 |
| Under prophylaxis | ||
| No | 1 (Reference) | |
| Yes | 0.72 (0.39-1.35) | .31 |
| Follow-up time (per year) | 0.89 (0.79-1.01) | .07 |
Multivariable analysis using mixed-effects Poisson regression model with robust standard errors. Only patients with known prophylaxis status (83 of 87 patients with hTTP) were included.
Characteristics, severity, and treatment of reported acute TTP episodes during follow-up
The median time between onset of symptoms of an acute episode and start of treatment was 0.0 days (range, 0.0-4.0 days), with 79.4% (104 of 131) of acute episodes immediately recognized (Table 5).
Specification, clinical presentation, and treatment of acute TTP episodes during follow-up
| Variable . | All episodes . | Prophylactic treatment . | P* . | ||||
|---|---|---|---|---|---|---|---|
| . | . | No . | Yes . | ||||
| N . | Median [min, lq, uq, max] or n (%) . | N . | Median [min, lq, uq, max] or n (%) . | N . | Median [min, lq, uq, max] or n (%) . | ||
| Onset of symptoms and medical care | |||||||
| Interval, d | 131 | 0.0 [0.0, 0.0, 0.0, 4.0] | 40 | 0.0 [0.0, 0.0, 0.0, 1.0] | 91 | 0.0 [0.0, 0.0, 0.0, 4.0] | .09 |
| On the same day | 131 | 104 (79%) | 40 | 35 (88%) | 91 | 69 (76%) | .16 |
| Duration of an episode, d | 128 | 4.0 [0.0, 2.0, 5.0, 43] | 39 | 4.0 [0.0, 3.0, 6.0, 36] | 89 | 3.0 [0.0, 2.0, 5.0, 43] | .06 |
| Trigger(s) of acute TTP episode† | 131 | 87 (66%) | 40 | 31 (77%) | 91 | 56 (62%) | .11 |
| Infection | 86 | 65 (76%) | 31 | 28 (90%) | 55 | 37 (67%) | .019 |
| Alcohol consumption | 86 | 5 (5.8%) | 31 | 1 (3.2%) | 55 | 4 (7.3%) | .65 |
| Pregnancy | 87 | 6 (6.9%) | 31 | 0 (0%) | 56 | 6 (11%) | .08 |
| Other | 85 | 11 (13%) | 30 | 3 (10%) | 55 | 8 (15%) | .74 |
| Clinical presentation† | |||||||
| Fever | 124 | 28 (23%) | 37 | 9 (24%) | 87 | 19 (22%) | .82 |
| Petechiae or other bleeding | 128 | 56 (44%) | 40 | 17 (43%) | 88 | 39 (44%) | 1.00 |
| Gastrointestinal symptoms | 128 | 38 (30%) | 40 | 10 (25%) | 88 | 28 (32%) | .53 |
| Jaundice | 128 | 18 (14%) | 40 | 7 (17%) | 88 | 11 (13%) | .58 |
| Dark urine | 127 | 15 (12%) | 40 | 3 (7.5%) | 87 | 12 (14%) | .39 |
| Presence of acute renal failure | 129 | 9 (7.0%) | 40 | 1 (2.5%) | 89 | 8 (9.0%) | .27 |
| Neurologic symptoms | 129 | 28 (22%) | 40 | 9 (22%) | 89 | 19 (21%) | 1.00 |
| Epileptic seizure | 28 | 0 (0%) | 9 | 0 (0%) | 19 | 0 (0%) | — |
| Transient ischemic attack | 28 | 9 (32%) | 9 | 5 (56%) | 19 | 4 (21%) | .10 |
| Stroke | 28 | 11 (39%) | 9 | 3 (33%) | 19 | 8 (42%) | 1.00 |
| Other | 28 | 19 (68%) | 9 | 8 (89%) | 19 | 11 (58%) | .20 |
| Treatment of acute episode‡ | 130 | 119 (92%) | 40 | 35 (88%) | 90 | 84 (93%) | .31 |
| Fresh frozen plasma | 119 | 73 (61%) | 35 | 28 (80%) | 84 | 45 (54%) | .008 |
| Solvent/detergent plasma | 119 | 34 (29%) | 35 | 4 (11%) | 84 | 30 (36%) | .008 |
| Cryo-poor plasma | 119 | 8 (6.7%) | 35 | 0 (0%) | 84 | 8 (10%) | .10 |
| Plasma-derived factor VIII product | 119 | 10 (8.4%) | 35 | 0 (0%) | 84 | 10 (12%) | .033 |
| Other | 119 | 6 (5.0%) | 35 | 3 (8.6%) | 84 | 3 (3.6%) | .36 |
| Variable . | All episodes . | Prophylactic treatment . | P* . | ||||
|---|---|---|---|---|---|---|---|
| . | . | No . | Yes . | ||||
| N . | Median [min, lq, uq, max] or n (%) . | N . | Median [min, lq, uq, max] or n (%) . | N . | Median [min, lq, uq, max] or n (%) . | ||
| Onset of symptoms and medical care | |||||||
| Interval, d | 131 | 0.0 [0.0, 0.0, 0.0, 4.0] | 40 | 0.0 [0.0, 0.0, 0.0, 1.0] | 91 | 0.0 [0.0, 0.0, 0.0, 4.0] | .09 |
| On the same day | 131 | 104 (79%) | 40 | 35 (88%) | 91 | 69 (76%) | .16 |
| Duration of an episode, d | 128 | 4.0 [0.0, 2.0, 5.0, 43] | 39 | 4.0 [0.0, 3.0, 6.0, 36] | 89 | 3.0 [0.0, 2.0, 5.0, 43] | .06 |
| Trigger(s) of acute TTP episode† | 131 | 87 (66%) | 40 | 31 (77%) | 91 | 56 (62%) | .11 |
| Infection | 86 | 65 (76%) | 31 | 28 (90%) | 55 | 37 (67%) | .019 |
| Alcohol consumption | 86 | 5 (5.8%) | 31 | 1 (3.2%) | 55 | 4 (7.3%) | .65 |
| Pregnancy | 87 | 6 (6.9%) | 31 | 0 (0%) | 56 | 6 (11%) | .08 |
| Other | 85 | 11 (13%) | 30 | 3 (10%) | 55 | 8 (15%) | .74 |
| Clinical presentation† | |||||||
| Fever | 124 | 28 (23%) | 37 | 9 (24%) | 87 | 19 (22%) | .82 |
| Petechiae or other bleeding | 128 | 56 (44%) | 40 | 17 (43%) | 88 | 39 (44%) | 1.00 |
| Gastrointestinal symptoms | 128 | 38 (30%) | 40 | 10 (25%) | 88 | 28 (32%) | .53 |
| Jaundice | 128 | 18 (14%) | 40 | 7 (17%) | 88 | 11 (13%) | .58 |
| Dark urine | 127 | 15 (12%) | 40 | 3 (7.5%) | 87 | 12 (14%) | .39 |
| Presence of acute renal failure | 129 | 9 (7.0%) | 40 | 1 (2.5%) | 89 | 8 (9.0%) | .27 |
| Neurologic symptoms | 129 | 28 (22%) | 40 | 9 (22%) | 89 | 19 (21%) | 1.00 |
| Epileptic seizure | 28 | 0 (0%) | 9 | 0 (0%) | 19 | 0 (0%) | — |
| Transient ischemic attack | 28 | 9 (32%) | 9 | 5 (56%) | 19 | 4 (21%) | .10 |
| Stroke | 28 | 11 (39%) | 9 | 3 (33%) | 19 | 8 (42%) | 1.00 |
| Other | 28 | 19 (68%) | 9 | 8 (89%) | 19 | 11 (58%) | .20 |
| Treatment of acute episode‡ | 130 | 119 (92%) | 40 | 35 (88%) | 90 | 84 (93%) | .31 |
| Fresh frozen plasma | 119 | 73 (61%) | 35 | 28 (80%) | 84 | 45 (54%) | .008 |
| Solvent/detergent plasma | 119 | 34 (29%) | 35 | 4 (11%) | 84 | 30 (36%) | .008 |
| Cryo-poor plasma | 119 | 8 (6.7%) | 35 | 0 (0%) | 84 | 8 (10%) | .10 |
| Plasma-derived factor VIII product | 119 | 10 (8.4%) | 35 | 0 (0%) | 84 | 10 (12%) | .033 |
| Other | 119 | 6 (5.0%) | 35 | 3 (8.6%) | 84 | 3 (3.6%) | .36 |
n, number of episodes in each category; N, number of episodes with information available.
Comparison of groups on and not on prophylactic treatment.
Listings are not additive; multiple entries are possible.
For 11 of the 131 acute episodes, patients received no treatment; for 1 episode, no treatment details were documented.
Plasma therapy was specifically installed to treat 92% of acute episodes, also in patients on regular prophylaxis. Treatment of choice was fresh frozen plasma (n = 73) or solvent detergent plasma (n = 34), followed by plasma-derived factor VIII concentrates (n = 10). The remaining 14 episodes were treated with cryo-poor plasma or other blood products (Table 5). Of note, 11 acute episodes were left untreated, as the patients’ treating physicians did not see a need for immediate or specific treatment of the episode. Nevertheless, 5 patients were subsequently started on prophylactic plasma treatment following the episode. The median duration of an episode to recovery was 4.0 days (range, 0.0-43 days).
Major clinical presentations during acute episodes were similar between patients on or not on plasma prophylaxis (Table 5). Although 90% of acute episodes in patients not under plasma prophylaxis were triggered by infections, the corresponding number in patients receiving regular plasma therapy was 67% (P = .019).
To assess disease severity during follow-up, we introduced a score for the acute episodes (Table 1); the lowest score described mild episodes, whereas the highest score of 4 was reserved for fatal outcome of typical TTP episodes and/or death from an acute cerebrovascular or cardiovascular event, or maternal death in the context of a pregnancy. Four patients died during prospective follow-up (Figure 4A). Although the cause of 1 death remained unknown (and was not categorized as an event; patient #62) (Figure 2), the other 3 deaths were considered category 4 events. These three deaths occurred in patients aged 33 years (#23; cause of death, large cerebral infarction), 39 years (#38; heart failure), and 56 years (#81; lethal arrhythmia with asystole during sepsis) who had experienced 12, 5, and 6 acute episodes before enrollment, respectively. Patients #23 and #38 were on plasma prophylaxis at the time of death, and patient #81 had stopped regular plasma infusions four months before his death. Although patient #23 had no other TTP episodes during prospective follow-up, patients #38 and #81 both had one event with a score of 3 before death. Their calculated annual incidence rates were 0.59, 0.34, and 0.36, respectively (Figure 4B).
Severity of acute TTP episodes and survival of patients with hTTP during prospective follow-up. (A) Kaplan-Meier curve for overall survival of 87 patients with hTTP during prospective follow-up, during which 4 male patients died. Causes of death were: large cerebral infarction in a 33-year-old, heart failure at age 38 years, lethal arrhythmia with asystole during sepsis at age 56 years, and death of unknown cause at age 79 years. According to the treating physicians, none of the patients at the time of death was considered to have an acute TTP episode. Still, 3 of the 4 deaths were categorized as fatal outcome of cerebrovascular or cardiovascular events (acute hTTP episode, severity score 4). (B) The severity of acute TTP episodes are graded as follows (Table 1): score 1, mild; score 2, moderate, usually temporary clinical symptoms; score 3, severe, clinical symptoms producing often permanent morbidity; and score 4, fatal outcome of typical TTP episode and/or death of cerebrovascular or cardiovascular event, or a female patient’s death during pregnancy.
Severity of acute TTP episodes and survival of patients with hTTP during prospective follow-up. (A) Kaplan-Meier curve for overall survival of 87 patients with hTTP during prospective follow-up, during which 4 male patients died. Causes of death were: large cerebral infarction in a 33-year-old, heart failure at age 38 years, lethal arrhythmia with asystole during sepsis at age 56 years, and death of unknown cause at age 79 years. According to the treating physicians, none of the patients at the time of death was considered to have an acute TTP episode. Still, 3 of the 4 deaths were categorized as fatal outcome of cerebrovascular or cardiovascular events (acute hTTP episode, severity score 4). (B) The severity of acute TTP episodes are graded as follows (Table 1): score 1, mild; score 2, moderate, usually temporary clinical symptoms; score 3, severe, clinical symptoms producing often permanent morbidity; and score 4, fatal outcome of typical TTP episode and/or death of cerebrovascular or cardiovascular event, or a female patient’s death during pregnancy.
Most frequent were acute TTP episodes of severity score 1 (102 of 131), which occurred primarily in patients with annual incidence rates >0.5 to 1 and >1. Ten acute episodes with a severity score of 3, and the potential for important morbidity and sequelae, were documented in 9 patients (4 female patients) (Figure 4B); seven of these patients had had comorbidities and sequelae at enrollment resulting from events with a score of 3 in the past before enrollment into the registry. Nine of the 10 events with scores of 3 were strokes.
Incidence of acute episodes in relation to ADAMTS13 activity and ADAMTS13 genotype
To investigate whether residual ADAMTS13 activity at baseline had an impact on the annual incidence rate of acute episodes, we considered only patients (n = 70) having their baseline ADAMTS13 activity measured at Bern University Hospital by using the modified FRETS-VWF73 assay.25,26 Because of unknown plasma prophylaxis status, we excluded one patient from this analysis. Of the 69 remaining patients, 49 (71%) patients had a very severe ADAMTS13 deficiency (ADAMTS13 activity ≤1%), whereas 20 had some residual ADAMTS13 activity (between 2% and 10%). An annual incidence rate of acute episodes ≤0.5 was recorded in 80% (16 of 20) of patients with residual ADAMTS13 activity at baseline, and in 67.3% (33 of 49) of patients with ADAMTS13 activity ≤1% (Figure 3D-E).
A large number of patients in our follow-up study were homozygous (n = 27) or compound heterozygous (n = 18) carriers of the ADAMTS13 c.4143_4144dupA mutation that in homozygotes is associated with no measurable ADAMTS13 activity in the circulation.8 The ADAMTS13 c.4143_4144dupA homozygotes were all ≥18 years of age and had a median annual incidence rate of acute episodes of 0.25 (range, 0.0-1.02), which was similar to that of the adult patients carrying only one, or no c.4143_4144dupA allele (supplemental Figure 1A).
Twelve patients (9 female patients) were compound heterozygous carriers of the ADAMTS13 c.3178C>T (p.R1060W) mutation that is associated with residual ADAMTS13 activity of 3% to 8% per allele.6,8 Its carriers often present with an adult onset of overt hTTP, especially during pregnancy.14-16 Nine of the compound heterozygous p.R1060W carriers had a low recurrence rate (annual incidence rate ≤0.5); the other three had annual incidence rates of 0.7 (n = 2) and 0.9 (n = 1), respectively (supplemental Figure 1B).
In the registry, there are 13 hTTP patients from 6 families who have an affected family member who participates in the registry (4 pairs and 1 trio of siblings; and one father–daughter pair); the brackets in Figure 2 link the respective family members. Annual incidence rates of acute episodes between affected siblings were similar in 4 families and different in the family with 3 affected siblings and in the father–daughter pair (supplemental Table 4).
Outcome of pregnancies in hTTP patients during follow-up
During follow-up, 5 patients (#10, #12, #16, #76, and #77) (Figure 2) had 13 pregnancies; one of them had ended in miscarriage (#16), four were ongoing at the last follow-up visit up to December 2019, and 8 pregnancies had resulted in live births of healthy infants who all survived (supplemental Figure 2). Deliveries were categorized according to the classification recommended by the World Health Organization28,29 for 6 pregnancies at term (37-41 weeks of gestation), whereas in 2 instances, delivery was very preterm and late preterm (30 and 36 weeks of gestation, respectively). During the pregnancies, all patients received regular plasma treatment, which had been established before becoming pregnant in three (patients #10, #12, and #16), whereas 2 women were started on regular plasma infusions immediately when pregnancies were confirmed (patients #76 and #77). Plasma prophylaxis was closely monitored and volumes adapted with increasing body weight and raised to keep the platelet count in the individual patient’s normal range.
Discussion
hTTP is an ultra-rare blood disorder with a prevalence of 0.5 to 2 cases per million.3 Despite increasing awareness over the past 2 decades, long-term outcome data are largely lacking, and available information often stems from retrospective data collections5,7,8 and single case reports that are a snapshot in time. The current study is the first large report on 87 patients with hTTP prospectively followed up in the Hereditary TTP Registry. Over 371 person-years, 44 patients had no acute TTP episodes, whereas 43 patients experienced 131 acute TTP episodes, corresponding to an overall annual incidence rate of acute episodes of 0.35 (95% CI, 0.29-0.42) (Table 3).
Of the 131 documented acute TTP episodes, 88 (67.2%) occurred in women, and 75 (57.2%) occurred in 15 patients aged <18 years at enrollment. Multivariable analysis considering age, sex, use of plasma prophylaxis, and time in follow-up showed that age (not female sex) was the driver of the acute episodes. Because vWF plasma levels rise with age,30 and even ADAMTS13 activity values in the lower normal range are associated with an increased risk of ischemic stroke, coronary heart disease, and cardiovascular mortality,31,32 we had hypothesized that more acute TTP episodes would be documented in older patients with hTTP. On the contrary, we observed a high number of acute events in young patients. Indeed, 35 (26.7%) episodes were documented in seven patients aged 2 to 7 years, the majority of them occurring in the context of mild to moderate infections (Figure 2B). These observations suggest that early childhood is a vulnerable period in the life of hTTP patients and are in line with recent small case series on pediatric patients with hTTP.11,12,17,18 Together, this stresses the importance of timely recognition of child-onset hTTP.
An important gap exists between overt disease onset and clinical diagnosis of hTTP, which potentially contributed to the high prevalence of comorbidities at enrollment, particularly of arterial thrombotic events.8 Despite awareness and an established diagnosis of hTTP in participants of the Hereditary TTP Registry, as well as regular prophylaxis in the majority of them, the currently used plasma infusion regimens are insufficient to prevent acute TTP episodes in a significant number of patients. Indeed, while receiving plasma prophylaxis, 31 of 61 patients with hTTP experienced 91 of 131 hTTP episodes (in 254 prospective patient-years; annual incidence rate of acute episodes of 0.36 [95% CI, 0.29-0.44], which was only borderline better than without prophylaxis) (Table 3). In the majority of patients, regular plasma prophylaxis followed the recommended dose (10-15 mL/kg) and interval (administration every 2-3 weeks), which projects to a plasma volume of ≤400 mL/kg per year. Higher doses were administered to one-fifth of patients who all had annual incidence rates of acute episodes ≤1.
The highest amounts of plasma were administered to female patients with hTTP during the final weeks of pregnancies with doses as high as 30 to 40 mL/kg per week (projecting to ∼1500-2000 mL/kg per year). In addition, plasma prophylaxis during pregnancies was closely monitored and adapted to keep the platelet counts in the patient’s individual normal range. This approach was effective and associated with delivery at term in 75% of pregnancies and a live birth rate of 89%, suggesting an improved outcome than previously observed.13-15 These are encouraging data and suggest that patients with hTTP can have successful pregnancies when regular plasma prophylaxis is installed early, monitored, and adapted (eg, by reducing the interval between administrations, or by increasing the volume given) when necessary. A multidisciplinary approach involving treating physician, hematologist, obstetrician, and neonatologist is suggested for the best outcomes.33
Chronic ADAMTS13 deficiency as a relevant cardiovascular and cerebrovascular risk factor31,32 is present in all patients with hTTP, even in those under strict plasma prophylaxis. Therefore, other existing cardiovascular risk factors should be managed rigorously, and long-term treatment with aspirin should be considered.34,35
More than 150 different causative ADAMTS13 mutations have been described in hTTP, ADAMTS13 c.4143_4144dupA and ADAMTS13 c.3178C>T (p.R1060W) being the most prevalent.7-9,11,14,15,22 Of the 87 patients with hTTP in prospective follow-up, 18 carried one and 27 carried two ADAMTS13 c.4143_4144dupA alleles; 12 were carriers of one ADAMTS13 c.3178C>T (p.R1060W) allele. We detected no dosage effect for the number of ADAMTS13 c.4143_4144dupA alleles, although this analysis may have been masked, as all but one of the 27 homozygotes were ≥18 years of age at enrollment and thus adults during follow-up (supplemental Figure 1A). The 12 carriers of one p.R1060W allele had ADAMTS13 activities ranging from 1% to 9% and a low incidence rate of acute episodes.
Residual ADAMTS13 activity has been reported to modulate the clinical phenotype in hTTP.6,11 Of the 69 patients with hTTP with information on plasma prophylaxis and baseline ADAMTS13 activity determined by using the modified FRETS-VWF73 assay,25,26 residual baseline ADAMTS13 activity (range, 2%-10%) was documented in 20 patients, whereas 49 had a very severe ADAMTS13 deficiency (≤1%) (Figure 3D-E). An annual incidence rate of acute episodes of ≤0.5 was observed in 80% (16 of 20) of patients with residual ADAMTS13 activity at baseline compared with 67.3% (33 of 49) of patients having ADAMTS13 activity ≤1%. This outcome suggests at most a mild effect of residual baseline ADAMTS13 activity on the clinical phenotype. In two-thirds of all episodes, a potential trigger was identified, which was an infection in 75% of cases.
The clinical presentation of acute TTP episodes in our cohort of 87 patients with hTTP was variable but produced the most common signs and symptoms published in the literature and ranged from laboratory abnormalities only to clinical manifestations that produced lasting sequelae and 3 deaths. Presentations were similar in patients with or without plasma prophylaxis (Table 5).
In the past, there have been attempts to assess the severity of hTTP based on accumulated comorbidities and/or special events experienced (ie, exchange blood transfusion in the neonatal period),6,8,9,11 which proved not suitable for the prospective evaluation of hTTP patients. We have developed a scoring system (Table 1) and assessed the severity of all 131 acute hTTP episodes. Most frequent were episodes (102 of 131) of severity score 1, which occurred primarily in patients with annual incidence rates >0.5 (Figure 4B). Given their temporary effect, they have often not prompted the treating physician to change overall treatment. We observed no typical TTP episode with fatal outcome; however, 3 of the 4 deaths during follow-up were due to premature cerebrovascular or cardiovascular events (Figure 4A).
Our study has several limitations. Forty-four patients enrolled in the registry from Japan had to be temporarily excluded from the analysis because of new consent requirements (Figure 1). Next, the definition of an acute TTP episode as an illness for which the patient required and/or received medical care in addition to regular prophylactic treatment may lead to underreporting of acute episodes. Many patients with hTTP are familiar with ambiguous symptoms such as headaches, irritation, difficulties with concentration, abdominal discomfort, and subfebrile temperatures that resolve within a few hours after plasma infusion and are thus an indication of non-overt TTP episodes. Patients have grown accustomed to these ailments and seldom seek medical care. We have no possibility of documenting all these non-overt or smoldering TTP episodes, which are also difficult to distinguish from headaches or abdominal discomfort of other reasons. We have restricted disease activity to acute overt TTP episodes and accept that the true burden of the disease may have been underestimated. Moreover, the very variable clinical presentation in terms of signs and symptoms, as well as in terms of their duration, gives the treating physicians great leeway to interpret and document the findings.
There are no evidence-based recommendations or guidelines on when to start or how to perform prophylactic treatment in patients with hTTP. In the case of registry patients, the treating physicians decide on the initiation and the modalities of prophylaxis. This approach potentially creates a site-wise selection bias in patients on prophylaxis. Moreover, the documented prophylaxis regimens were variable between different patients but also in a number of patients over time. Our data set is not yet sufficiently large to analyze the different prophylaxis regimens in-depth. Because the study is ongoing, the ever-growing prospective follow-up will increase robustness of data in the future.
In conclusion, the Hereditary TTP Registry has provided for the first time prospective data of 87 confirmed hTTP patients. The overall annual incidence rate of acute episodes was considerably lower than the annual bleeding rate in patients with hemophilia,36,37 another inherited disorder originally treated with plasma infusions. This data needs to be taken into account when designing prophylaxis and treatment studies with recombinant ADAMTS13.
The efficacy of currently used prophylaxis regimens (infusion of 10-15 mL plasma/kg every 2-3 weeks) is often insufficient to prevent acute episodes. This was particularly true for young children and women. Once recombinant ADAMTS13 becomes available38 and regular home treatment will become possible, the decision to treat patients with hTTP prophylactically will be less difficult, and applied doses will likely be more efficient than the current volume-wise limited plasma regimens.
Requests for original data may be submitted to the corresponding author (Johanna A. Kremer Hovinga; e-mail: johanna.kremer@insel.ch).
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank the many sites and patients from all over the world who participate in this project. The responsible physicians and academics at the different sites participating in this project are listed in supplemental Appendix A. The contribution of Marienn Réti (Department of Hematology and Stem Cell Transplantation, National Institute of Hematology and Infectious Diseases, Central Hospital of Southern Pest, Budapest, Hungary), Renata Tomaszewska and Maria Szczepanska (Medical University of Silesia in Katowice, Zabrze, Poland), and Beata Baran, Edyta Odnoczko, and Ksenia Bykowska (Institute of Hematology and Transfusion Medicine, Warsaw, Poland) in laboratory investigation and/or patient care is gratefully acknowledged.
The Hereditary TTP Registry has received support through grants from the Swiss National Science Foundation (grant 310030-185233), the Mach-Gaensslen Foundation Switzerland, the ISTH 2007 Presidential Fund, the GTH Congress President Fund, the NFG Foundation, and a research grant from Baxalta US Inc., member of the Takeda group of companies. This work was further supported in part by grants-in-aid from the Ministry of Health, Labor and Welfare of Japan and the Higher Education Institutional Excellence Program of the Ministry of Human Capacities in Hungary within the framework of the molecular biology thematic program of Semmelweis University, Budapest, Hungary.
Authorship
Contribution: E.T., L.B., B.L., and J.A.K.H. designed the study, which was approved according to the registry project guidelines by K.D.F., J.N.G., I.H., P.N.K., M.M., A.S.v.K., B.L., and J.A.K.H; E.T., I.A.-H., Z.C., M.G.-K., K.A.J., C.R.L., Z.P., G.S., and J.W. collected data; E.T., L.B., B.L., and J.A.K.H. analyzed data; E.T. and J.A.K.H. wrote the paper and had editorial support from J.N.G. and B.L.; and all authors edited the final manuscript.
Conflict-of-interest disclosure: P.N.K. received consultancy and advisory board fees, speaker honoraria, and travel grants from Ablynx/Sanofi, Alexion, and Shire/Takeda. M.M. is a member of the advisory board of Takeda, Alexion, and Sanofi. A.S.v.K. is a member of advisory board of Sanofi Genzyme. J.W. received grant support or lectures honoraria from Alexion, Alnylam Pharmaceuticals, Baxalta, CSL Behring, Ferring Pharmaceuticals, Novo Nordisk, Octapharma, Rigel Pharmaceuticals, Roche, Sanofi/Genzyme, Shire/Takeda, Sobi, and Werfen. B.L. is chairman of the data safety monitoring committees for the Baxalta 281102 study (recombinant ADAMTS13 in congenital TTP) and for the Takeda SHP655-201 study (recombinant ADAMTS13 in acquired TTP), now both run by Takeda; is a member of the advisory board of Ablynx, now part of Sanofi, for the development of caplacizumab; and received congress travel support and/or lecture fees from Baxter, Ablynx, Alexion, Siemens, Bayer, Roche, and Sanofi. J.A.K.H. is a member of the advisory board of Shire, member of the Takeda group of companies, for the development of recombinant ADAMTS13, and of Ablynx, now part of Sanofi for the development of caplacizumab. The remaining authors declare no competing financial interests.
Correspondence: Johanna A. Kremer Hovinga, Department of Hematology and Central Hematology Laboratory, Inselspital, Bern University Hospital, University of Bern, Freiburgstrasse 18, CH-3010 Bern, Switzerland; e-mail: johanna.kremer@insel.ch.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal