In this issue of Blood, Iannello et al report that phosphatidylinositol 3-kinase (PI3K) inhibitors can sensitize samples from patients with Richter syndrome (RS) to BCL2 inhibitors by regulating the expression of antiapoptotic molecules via glycogen synthase kinase 3 β (GSK3β). The authors have thus provided a rationale for a novel drug combination to treat patients with chronic lymphocytic leukemia (CLL) and RS.1
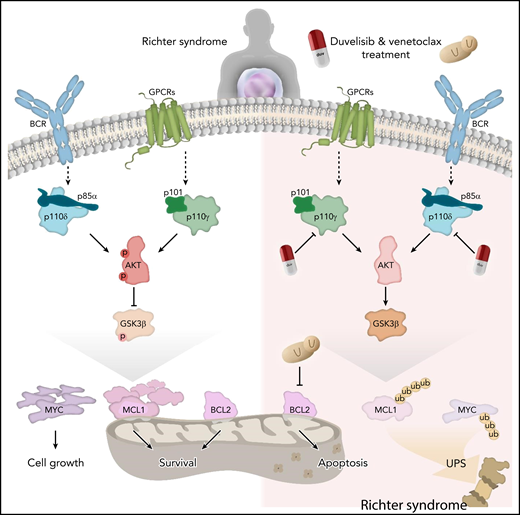
RS cells show expression and activation of components of the PI3K-AKT axis that can be targeted by duvelisib, which leads to activation of GSK3β and results in MCL1 and MYC ubiquitination and degradation via the proteasome. Downregulation of MCL1 and MYC sensitizes RS cells to venetoclax-mediated intrinsic apoptosis. GPCR, G-protein–coupled receptor; p, phosphorylation; ub, ubiquitination; UPS, ubiquitin proteasome system. Figure designed by Matteo Oliverio. The figure has been adapted from Iannello et al that begins on page 3378.
RS cells show expression and activation of components of the PI3K-AKT axis that can be targeted by duvelisib, which leads to activation of GSK3β and results in MCL1 and MYC ubiquitination and degradation via the proteasome. Downregulation of MCL1 and MYC sensitizes RS cells to venetoclax-mediated intrinsic apoptosis. GPCR, G-protein–coupled receptor; p, phosphorylation; ub, ubiquitination; UPS, ubiquitin proteasome system. Figure designed by Matteo Oliverio. The figure has been adapted from Iannello et al that begins on page 3378.
Development of RS is one of the most dire complications of CLL because it is associated with poor clinical outcome with no proven treatment that leads to sustained remission. RS occurs in 5% to 10% of all patients with CLL that have the development of an aggressive lymphoma, mainly diffuse large B-cell lymphoma (DLBCL) and, in rare cases, Hodgkin lymphoma.2 In more than 80% of these cases, DLBCL-RS is clonally related to CLL.2 Clonally related DLBCL-RS has an especially poor outcome with a median overall survival (OS) of 3 to 12 months, whereas clonally unrelated DLBCL has an OS of 5 years.3 The molecular basis of transformation is not fully understood. It was recently shown that active AKT signaling triggers the transformation of CLL toward RS via overactivation of the NOTCH1 pathway.4 Indeed, patients with CLL who harbor high-risk molecular characteristics such as TP53 alterations or NOTCH1 mutations, have a higher risk of developing RS. In addition, alterations in MYC and losses of CDKN2A are mostly found after transformation and were found in patients after chemoimmunotherapy with venetoclax and ibrutinib.5-7 The impact of previous treatment on RS transformation is unclear, because almost half of patients with RS are therapynaive at diagnosis.
As in many malignancies with diverse and complex genetic alterations, we have to ask whether our understanding of the mutational landscape is sufficient for understanding resistance to therapy and for providing a rationale for effective treatment options. In the study by Iannello et al, the researchers focused on protein and transcript expression of potential target molecules. They determined expression of PI3K catalytic (p110δ and p110γ) and regulatory (p85α and p101) subunits, phosphorylated and total BTK and AKT, and the apoptotic pathway proteins BCL2, MCL1, and c-Myc. p110δ and p85α subunits were homogeneously expressed at high levels in all 24 RS samples, whereas the catalytic p110γ and the regulatory p101 subunits displayed variable levels. This variable expression correlated with a different activation status of BTK and AKT. In addition, the authors report that 6 of 24 RS samples did not express detectable levels of BCL2. To evaluate the role of different expression patterns, elegant patient-derived xenograft (PDX) models were used to test treatment efficacy in vivo. Cells from 4 different patients with RS were transplanted subcutaneously or intravenously. Response to duvelisib and venetoclax was directly related to the expression level of their respective target molecules. The authors demonstrated that the efficacy of duvelisib and venetoclax in RS cells relies on the concomitant inactivation of MCL1, MYC, and BCL2. Treatment with duvelisib activated GSK3β, resulting in Mcl-1/c-Myc ubiquitination and degradation via the proteasome (see figure). In this scenario, the simultaneous inhibition of BCL2 further sustains RS cell apoptosis. The work of Iannello et al provides, for the first time, proof of concept of the efficacy of dual targeting of PI3K-δ/γ and BCL2 in RS.
It would be of interest to test whether treatment with other PI3K inhibitors such as idelalisib or copanlisib can achieve comparable results. Copanlisib, which has proven efficacy in DLBCL models and is currently being evaluated in a trial with nivolumab, might be a very promising candidate (NCT03884998).8 Furthermore, the trial data indicate that some patients with RS might also qualify for combination treatment with BTK or BCL2 inhibitors, because BTK activation was seen in one of the RS models. Synergy between BTK or PI3K inhibitors and BH3 mimetics was shown to be mediated by regulation of the ceramide metabolism in primary CLL cells.9
The findings by Iannello et al stress that it is important to determine the expression profile of drug targets, because RS patients might be negative for BCL2 expression or the pAKT signal might be insufficient. Indeed, patients with low BCL2 but high MCL1 expression should be preferentially treated with MCL1 inhibitors. Actionable vulnerabilities should be explored by pharmacologic- or peptide-based BH3 profiling to determine the most suitable BH3 mimetic for each patient with CLL and RS. The authors have demonstrated that their PDX models can be used for validation experiments of novel drug combinations. PDX models also offer the advantage of being able to test treatment efficacy under physiological low oxygen tensions, which is a relevant factor for the efficacy of BH3 mimetics.10 The work by Iannello et al underscores the importance of suitable in vivo models that resemble human settings. However, to close the experimental circle, it needs to be shown that PDX models can be used as tools to build rational personalized treatment algorithms for RS. First results from a multicenter trial in RS for the duvelisib-venetoclax combination are awaited in 2022 (NCT03534323).
Based on the expression pattern and mode of action of PI3K and BCL2, simultaneous targeting of PI3K and BCL2 is a logical starting point for targeting RS. Although PI3K inhibition sensitizes CLL and RS cells toward intrinsic apoptosis (induced by venetoclax), it is intriguing to speculate that additional targeting of extrinsic apoptosis pathways might result in even deeper and longer-lasting responses. Indeed, we hold all of the necessary tools in our hands for conducting such trials to improve outcomes for our patients with CLL with RS.
Conflict-of-interest disclosure: L.P.F. received honoraria and travel grants from AbbVie, honoraria from Roche, and research grants from AbbVie, Roche, and Gilead.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal