In this issue of Blood, Vaisitti et al1 demonstrate that the receptor tyrosine kinase–like orphan receptor 1 (ROR1), which is expressed on the cells of various hematological malignancies and solid tumors, but not on normal adult tissues, is a potential therapeutic target for treatment of Richter transformation. Using a xenograft mouse model, they showed a significant therapeutic effect using the ROR1-directed antibody drug conjugate (ADC) VLS-101 (see figure).
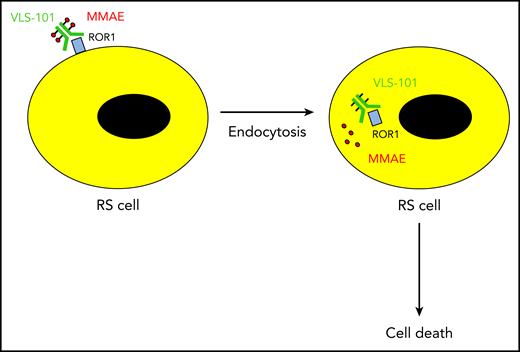
The ADC VLS-101 binds ROR1 on the surface of Richter syndrome (RS) cells. Upon rapid internalization of the ADC, the cytotoxin monomethyl auristatin E (MMAE) is released into the cytosol and kills the RS cell.
The ADC VLS-101 binds ROR1 on the surface of Richter syndrome (RS) cells. Upon rapid internalization of the ADC, the cytotoxin monomethyl auristatin E (MMAE) is released into the cytosol and kills the RS cell.
RS refers to the development of an aggressive lymphoma in patients with chronic lymphocytic leukemia (CLL).2 While diffuse large B cell lymphoma (DLBCL) is by far the most common histology, Hodgkin lymphoma and T-cell lymphomas can also occur. DLBCL-like RS carries a particularly dismal prognosis. The clonal relationship between the underlying CLL and the DLBCL provides the most important prognostic information. Patients with a clonally related DLBCL transformation show a median survival of only 14 months, whereas patients with clonally unrelated transformation have a significantly better prognosis, with a median survival of 5 years.3 Approximately 80% of patients with DLBCL-like RS have clonally related disease and, thus, a bad prognosis. Transformation while on therapy with targeted agents like ibrutinib or venetoclax seems to be associated with an even poorer outcome, with a median survival of only 3 months.4
The mainstay of therapy for RS is anthracycline-containing chemotherapy plus rituximab, with R-CHOP (rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone) and dose-adjusted EPOCH-R (etoposide, prednisone, vincristine, cyclophosphamide, doxorubicin plus rituximab) being the preferred regimens. Nonmyeloablative allogeneic hematopoietic stem cell transplantation is an option for a subpopulation of patients and significantly improves the clinical outcome when performed during the first remission.5 A number of novel agents (ibrutinib, venetoclax, pembrolizumab, nivolumab, and selinexor) have shown clinical activity but with disappointing remission durations. The optimal combination of classical immunochemotherapy and novel agents is currently being investigated in several clinical trials. Two recently reported studies have demonstrated promising results with chimeric antigen receptor T cells.6 Overall, the therapeutic options especially for clonally related RS remain very limited, and new therapeutic options are desperately needed.
ADCs are a novel set of anticancer agents with excellent activity in a variety of malignancies. They typically consist of a monoclonal antibody directed against a specific antigen on malignant cells that is linked to several molecules of a cytotoxic drug. Upon binding their respective antigen, ADCs are rapidly internalized and eventually release the cytotoxic agent into the cytosol of the tumor cell. A number of ADCs are currently approved for cancer therapy. Brentuximab vedotin is a CD30-directed ADC approved for the treatment of Hodgkin disease and systemic anaplastic large cell lymphoma.7 Trastuzumab emtansine is used in the treatment of Her-2/neu–positive breast cancer.8 Inotuzumab ozogamicin,9 which is directed against CD22, is approved for the treatment of relapsed and refractory acute lymphoblastic leukemia, whereas gemtuzumab ozogamicin, which targets CD33, is used in the first-line treatment of acute myeloid leukemia. Polatuzumab vedotin10 is a CD79b-directed ADC that significantly improves the clinical outcome in patients with DLBCL when used in combination with bendamustine and rituximab.
Vaisitti et al now report that ROR1 is expressed on the surface of RS cells and can be efficiently targeted using the ADC VLS-101, resulting in significant remissions and improvement of overall survival in a mouse model. The authors employed a xenotransplant mouse model to investigate tumors derived from patients with DLBCL-like RS with different expression levels of ROR1. In chronic lymphocytic leukemia cells, ROR1 binds to the noncanonical Wnt signaling member Wnt5a, resulting in the activation of different guanine exchange factors and subsequently phosphatidylinositol-3 kinase and Janus kinase signaling cascades, culminating in cell proliferation. A phase 1 study with naked antibody to inhibit the ROR1 pathway failed to show significant clinical activity. This prompted the group to create the ROR1-directed ADC VLS-101, which was used in this study. While the ROR1 pathway does not appear to be activated in RS cells, the investigators show that ROR1 can serve as a robust target for the ADC. The treatment of mice with transplanted human RS-like tumors with high or intermediated ROR1 expression resulted in robust tumor regressions and a significant survival improvement.
In summary, the study of Vaisitti et al shows promising data with respect to the antitumor activity of a novel ADC directed against ROR1 in human Richter transformation. A phase 1 clinical trial evaluating VLS-101 in patients with DLBCL-like RS is currently underway. This should provide information about the true clinical potential of this agent for this devastating disease.
Conflict-of-interest disclosure: The author declares no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal