Key Points
FIX variant CB 2679d-GT significantly outperformed the R338L-Padua variant after gene therapy.
Increase in the overall potency of the gene therapy vectors may allow for lower and potentially safer vector doses in future human trials.
Abstract
Sustained expression of therapeutic factor IX (FIX) levels has been achieved after adeno-associated viral (AAV) vector-based gene therapy in patients with hemophilia B. Nevertheless, patients are still at risk of vector dose-limiting toxicity, particularly liver inflammation, justifying the need for more efficient vectors and a lower dosing regimen. A novel increased potency FIX (designated as CB 2679d-GT), containing 3 amino acid substitutions (R318Y, R338E, T343R), significantly outperformed the R338L-Padua variant after gene therapy. CB 2679d-GT demonstrated a statistically significant approximately threefold improvement in clotting activity when compared with R338L-Padua after AAV-based gene therapy in hemophilic mice. Moreover, CB 2679d-GT gene therapy showed significantly reduced bleeding time (approximately fivefold to eightfold) and total blood loss volume (approximately fourfold) compared with mice treated with the R338L-Padua, thus achieving more rapid and robust hemostatic correction. FIX expression was sustained for at least 20 weeks with both CB 2679d-GT and R338L-Padua whereas immunogenicity was not significantly increased. This is a novel gene therapy study demonstrating the superiority of CB 2679d-GT, highlighting its potential to obtain higher FIX activity levels and superior hemostatic efficacy following AAV-directed gene therapy in hemophilia B patients than what is currently achievable with the R338L-Padua variant.
Introduction
Sustained therapeutic factor IX (FIX) and FVIII levels were attained after liver-directed gene therapy with adeno-associated viral (AAV) vectors in patients with hemophilia.1-4 However, a dose-dependent increase in hepatotoxicity associated with loss of transgene expression was frequently observed, possibly due to adaptive or innate immune responses.1-7 One approach to reduce hepatotoxicity is to use lower vector doses, warranting the use of more potent expression cassettes8,9 or gain-of-function FIX transgenes.9-12 Transgenes encoding longer-acting FIX-fusion proteins were also investigated but did not increase therapeutic efficacy in mice.13 A particular substitution at position 338 (designated as R338L-Padua), associated with thrombophilia,14 enhances FIX activity by fivefold to eightfold. Extensive preclinical and clinical experience with R338L-Padua gene therapy in hemophilia B revealed no increased thrombogenic or immunogenic risk.1,2,9-11 Molecular regulation of activation, inactivation, and cofactor dependence of R338L-Padua is similar to wild-type FIX but FVIIIa-dependent hyperactivity of R338L-Padua is the result of a faster rate of FX activation.15 Although the R338L-Padua transgene has been adopted in all currently ongoing hemophilia B trials, some patients still developed transaminitis that correlated with loss of FIX expression despite immune suppression.1 These observations substantiate a need for developing alternative FIX transgenes that boost FIX activity compared with R338L-Padua.
A next-generation FIX variant, dalcinonacog alfa (previously CB 2679d/ISU304), was designed using rational protein engineering and is being developed for routine prophylaxis. It is based on triplet substitutions (R318Y, R338E, and T343R) that increase catalytic activity, provide resistance to antithrombin inhibition, and improve affinity for activated FVIII (FVIIIa).16-19 These enhancements provided a 22-fold enhanced potency over wild-type FIX in a phase 1/2 clinical trial and enabled administration by subcutaneous injection.18,19, In the present study, we showed that this novel gain-of-function FIX variant can significantly outperform the R338L-Padua transgene following AAV gene therapy in hemophilic mice. The use of this novel CB 2679d-GT gene therapy approach requires extensive studies to address immunogenic and thrombogenic risks in immunocompetent hemophilic animals, which is addressed in the current study.
Study design
Additional information can be found in supplemental Methods (available on the Blood Web site). AAV plasmid vectors contained a liver-specific promoter (α1-antitrypsin [AAT]) to express a codon-optimized human FIX (co-hFIX) complementary DNA with the R338L Padua variant (pAAV-AAT-co-hFIX-R338L-Padua or R338L-Padua; Figure 1A and supplemental Figure 1A)9,10 or the CB 2679d-GT transgene that encodes a human FIX (hFIX) protein with 3 amino acid substitutions R318Y, R338E, T343R (pAAV-AAT-co-hFIX-CB2679d-GT or CB 2679d-GT; Figure 1B and supplemental Figure 1B). The corresponding self-complementary20 AAV particles were packaged using the AAV/DJ8 serotype,21 purified, characterized, and titered, as described22 (supplemental Figure 2). Adult hemophilia B mice (FIX−/−)23 were injected intravenously at the indicated AAV vector doses. FIX antigen levels were determined on plasma samples by enzyme-linked immunosorbent assay (ELISA) using known concentrations of purified full-length recombinant R338L-Padua and CB 2679d-GT proteins, as respective standards. FIX activity was determined by determining the clotting time using an activated partial thromboplastin time (aPTT) single-stage clotting assay. Phenotypic correction was assessed using the mouse tail-clip assay.24 Plasma samples were also checked for anti-FIX antibodies, aspartate aminotransferase, and alanine transaminase activity.9 Vector copy number and biodistribution were assessed by quantitative real-time polymerase chain reaction (PCR).9 Animal experiments were approved by the university’s Animal Ethics Committee.
Vector design and functional validation. (A-B) Schematic representation of the pAAV-AAT-co-hFIX-R338L-Padua (A), pAAV-AAT-co-hFIX-CB2679d-GT (B) plasmids used in this study. The liver-specific promoter (AAT) drives the (co-hFIX) complementary DNA with the Padua variant (ie, R338L-Padua) or a CB 2679d-GT transgene that encodes a hFIX with 3 amino acid substitutions (ie, R318Y, R338E, T343R). The minute virus of mouse (MVM) mini-intron and bovine growth hormone polyadenylation site bghpA) are also indicated. The expression cassettes were cloned into an scAAV backbone, flanked by the 5′ and 3′ AAV inverted terminal repeats (ITRs), as indicated. (C-E) The R338L-Padua and CB 2679d-GT vectors were injected into hemophilia B mice (FIX−/−) at doses of 1 × 109 vg per mouse (5 × 1010 vg/kg) (n = 6), 5 × 109 vg per mouse (2.5 × 1011 vg/kg) (n = 8), and 1 × 1010 vg per mouse (5 × 1011 vg/kg) (n = 8). The FIX antigen levels were determined at the indicated times after AAV administration by using a hFIX-specific ELISA at 1 × 109 vg per mouse (C), 5 × 109 vg per mouse (D), and 1 × 1010 vg per mouse (E). To measure CB 2679d-GT and R338L-Padua antigen levels by ELISA and exclude any potential bias, FIX standard curves were constructed with known amounts of the purified recombinant CB 2679d-GT or R338L-Padua, respectively. (F-H) The FIX activity was determined as a measure of clotting time using an aPTT assay for the doses 1 × 109 vg per mouse (n = 6) (F), 5 × 109 vg per mouse (n = 8) (G), and 1 × 1010 vg per mouse (n = 8) (H). Vehicle-injected hemophilia B mice (FIX−/−) (n = 7) and C57BL/6 normal control (n = 4) (dotted line) were used as controls. (I-L) Mouse plasma samples were also used to determine the FIX activity (units per mL) and specific activity (units per mg) calculated based on a single-stage, aPTT-based factor IX clotting assay with WHO FIX standard (09/172) and the FIX antigen levels shown above. Two vector doses were used 5 × 109 vg per mouse (I,K) and 1 × 1010 vg per mouse (J,L). Results are presented as mean ± standard error of the mean. *P < .05; **P < .01; ***P < .001 (Student t test); NS, not significant (P > .1).
Vector design and functional validation. (A-B) Schematic representation of the pAAV-AAT-co-hFIX-R338L-Padua (A), pAAV-AAT-co-hFIX-CB2679d-GT (B) plasmids used in this study. The liver-specific promoter (AAT) drives the (co-hFIX) complementary DNA with the Padua variant (ie, R338L-Padua) or a CB 2679d-GT transgene that encodes a hFIX with 3 amino acid substitutions (ie, R318Y, R338E, T343R). The minute virus of mouse (MVM) mini-intron and bovine growth hormone polyadenylation site bghpA) are also indicated. The expression cassettes were cloned into an scAAV backbone, flanked by the 5′ and 3′ AAV inverted terminal repeats (ITRs), as indicated. (C-E) The R338L-Padua and CB 2679d-GT vectors were injected into hemophilia B mice (FIX−/−) at doses of 1 × 109 vg per mouse (5 × 1010 vg/kg) (n = 6), 5 × 109 vg per mouse (2.5 × 1011 vg/kg) (n = 8), and 1 × 1010 vg per mouse (5 × 1011 vg/kg) (n = 8). The FIX antigen levels were determined at the indicated times after AAV administration by using a hFIX-specific ELISA at 1 × 109 vg per mouse (C), 5 × 109 vg per mouse (D), and 1 × 1010 vg per mouse (E). To measure CB 2679d-GT and R338L-Padua antigen levels by ELISA and exclude any potential bias, FIX standard curves were constructed with known amounts of the purified recombinant CB 2679d-GT or R338L-Padua, respectively. (F-H) The FIX activity was determined as a measure of clotting time using an aPTT assay for the doses 1 × 109 vg per mouse (n = 6) (F), 5 × 109 vg per mouse (n = 8) (G), and 1 × 1010 vg per mouse (n = 8) (H). Vehicle-injected hemophilia B mice (FIX−/−) (n = 7) and C57BL/6 normal control (n = 4) (dotted line) were used as controls. (I-L) Mouse plasma samples were also used to determine the FIX activity (units per mL) and specific activity (units per mg) calculated based on a single-stage, aPTT-based factor IX clotting assay with WHO FIX standard (09/172) and the FIX antigen levels shown above. Two vector doses were used 5 × 109 vg per mouse (I,K) and 1 × 1010 vg per mouse (J,L). Results are presented as mean ± standard error of the mean. *P < .05; **P < .01; ***P < .001 (Student t test); NS, not significant (P > .1).
Results and discussion
The AAV-AAT-co-hFIX-R338L-Padua (ie, R338L-Padua) (Figure 1A) and AAV-AAT-co-hFIX-CB2679d-GT (ie, CB 2679d-GT) (Figure 1B) vectors were produced at high titer (5.2 × 1012 to 1.3 × 1013 vector genomes [vg]/mL). FIX antigen levels were comparable in FIX-deficient mice injected with the CB 2679d-GT vector or R338L-Padua vector (Figure 1C-E). The differences were not significant across all time points at the lower vector doses of 1 × 109 or 5 × 109 vg per mouse and only a slight increase was apparent in the highest-dose cohort injected with the R338L-Padua vector compared with CB 2679d-GT. In contrast, a significant increase in FIX activity was evident in those mice that expressed the CB 2679d-GT transgene (1 × 109 vg per mouse, n = 6; 5 × 109 vg per mouse, n = 8; 1010 vg per mouse, n = 8) compared with R338L-Padua (1 × 109 vg per mouse, n = 6; 5 × 109 vg per mouse, n = 8; 1010 vg per mouse, n = 8) based on the shortening of clotting time in a 1-stage clotting assay (Figure 1F-H). Clotting times were significantly reduced in hemophilia B (FIX−/−) mice treated with CB 2679d-GT (5 × 109 vg per mouse and 1 × 1010 vg per mouse) compared with healthy mice (n = 4) (Figure 1G-H). Similarly, a sustained level of ∼4 to 6 IU/mL for CB 2679d-GT and ∼3 to 5 IU/mL for R338L-Padua (based on a World Health Organization [WHO] FIX standard) could be attained at the 2 highest vector doses, yielding a significant and sustained increase in specific FIX activity (units per mg) in those mice that expressed CB 2679d-GT compared with R338L-Padua (Figure 1I-L).
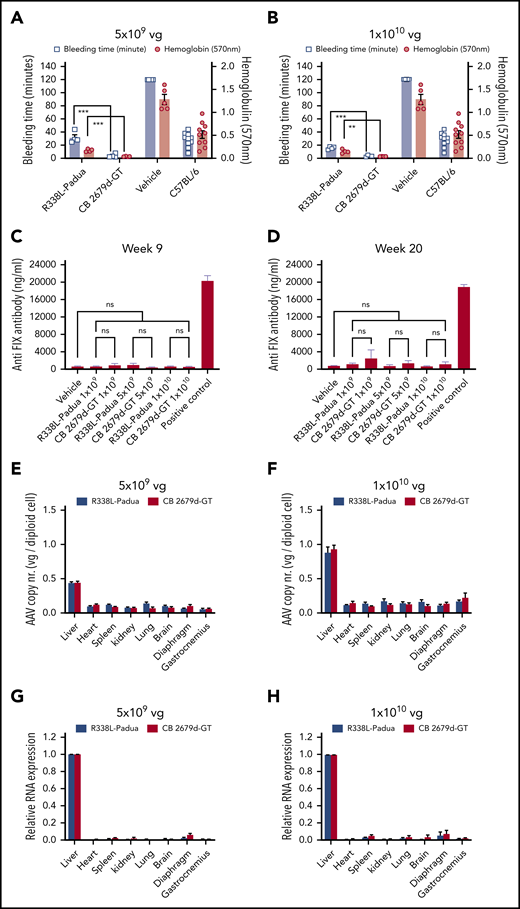
The in vivo efficacy of CB 2679d-GT and R338L-Padua was evaluated after 10 weeks of sustained expression in a tail-bleeding model. Notably, mice treated with CB 2679d-GT exhibited a significantly reduced (fivefold to eightfold) bleeding time (P < .001 at both doses) (Figure 2A-B) and blood loss (fourfold) (P < .001 at 5 × 109 vg per mouse, n = 4; and P < .01 at 1 × 1010 vg per mouse; n = 4) compared with mice treated with the R338L-Padua–containing vectors (n = 4 at each dose), thus achieving a more rapid and robust hemostatic correction of bleeding (Figure 2A-B). The greater reduction in bleeding time and blood loss was consistent with a more pronounced shortening of the clotting time in CB 2679d-GT–expressing mice (Figure 1F-H). Most importantly, the bleeding time and blood-loss volume was significantly reduced in CB 2679d-GT–expressing mice (P < .001 and P < .01, respectively, for both vector doses) compared with C57BL/6 wild-type mice (n = 10) (Figure 2A-B). The basis for the superior hemostatic potency of CB 2679d-GT compared with R338L-Padua is most likely related to the increase in catalytic activity, resistance to antithrombin inhibition, and improved affinity for activated FVIII observed with CB 2679d-GT16,17 resulting from the triplet R318Y, R338E, and T343R substitutions.
Functionality, safety, and specificity assessment of the vectors. (A-B) Phenotypic correction was assessed using a tail-clip model to measure the bleeding time and blood loss in the repeat experiment for experimental groups of R338L-Padua and CB 2679d-GT at the doses of 5 × 109 vg per mouse (n = 4) (A) and 1 × 1010 vg per mouse (n = 4) (B) compared with the vehicle control (n = 5) and C57BL/6 normal controls (n = 10). For humane reasons, vehicle control mice were euthanized after 120 minutes, although they were still bleeding at that time. Results are presented as mean ± standard error of the mean. *P < .05; **P < .01; ***P < .001 (Student t test); NS, not significant (P > .1) (C-D) The FIX-specific antibodies were determined by ELISA in hemophilia B mice (FIX−/−) injected with R338L-Padua and CB 2679d-GT at 3 doses (1 × 109vg per mouse, 5 × 109 vg per mouse and 1 × 1010 vg per mouse) at time points of 9 weeks (C) and 20 weeks (D) postinjection. *P < .05; **P < .01; ***P < .001 (Student t test); NS, not significant (P > .1). (E-H) AAV copy number in a panel of 8 organs from the mice injected with R338L-Padua or CB 2679d-GT at the 2 doses of 5 × 109 vg per mouse (E) and 1 × 1010 vg per mouse (F) were analyzed at 20 weeks postinjection using quantitative real-time PCR. The same mice were also analyzed for RNA expression in a panel of 8 organs by quantitative reverse transcription PCR to confirm that the RNA expression for both the vector groups was specific to liver 5 × 109 vg per mouse (G) and 1 × 1010 vg per mouse (H). The RNA expression of the transgene was normalized to mouse glyceraldehyde-3-phosphate dehydrogenase expression. Results are presented as mean ± standard error of the mean.
Functionality, safety, and specificity assessment of the vectors. (A-B) Phenotypic correction was assessed using a tail-clip model to measure the bleeding time and blood loss in the repeat experiment for experimental groups of R338L-Padua and CB 2679d-GT at the doses of 5 × 109 vg per mouse (n = 4) (A) and 1 × 1010 vg per mouse (n = 4) (B) compared with the vehicle control (n = 5) and C57BL/6 normal controls (n = 10). For humane reasons, vehicle control mice were euthanized after 120 minutes, although they were still bleeding at that time. Results are presented as mean ± standard error of the mean. *P < .05; **P < .01; ***P < .001 (Student t test); NS, not significant (P > .1) (C-D) The FIX-specific antibodies were determined by ELISA in hemophilia B mice (FIX−/−) injected with R338L-Padua and CB 2679d-GT at 3 doses (1 × 109vg per mouse, 5 × 109 vg per mouse and 1 × 1010 vg per mouse) at time points of 9 weeks (C) and 20 weeks (D) postinjection. *P < .05; **P < .01; ***P < .001 (Student t test); NS, not significant (P > .1). (E-H) AAV copy number in a panel of 8 organs from the mice injected with R338L-Padua or CB 2679d-GT at the 2 doses of 5 × 109 vg per mouse (E) and 1 × 1010 vg per mouse (F) were analyzed at 20 weeks postinjection using quantitative real-time PCR. The same mice were also analyzed for RNA expression in a panel of 8 organs by quantitative reverse transcription PCR to confirm that the RNA expression for both the vector groups was specific to liver 5 × 109 vg per mouse (G) and 1 × 1010 vg per mouse (H). The RNA expression of the transgene was normalized to mouse glyceraldehyde-3-phosphate dehydrogenase expression. Results are presented as mean ± standard error of the mean.
Given the presence of 3 distinct substitution mutations in CB 2679d-GT (ie, R318Y, R338E, and T343R), extensive immunological studies are required to assess the risk of anti-FIX antibodies, in comparison with R338L-Padua. None of the hemophilia B mice (FIX−/−) treated with the R338L-Padua developed anti-FIX antibodies (Figure 2C-D), which is consistent with previous reports.9-11 Similarly, the large majority of the hemophilia B mice (FIX−/−) treated with the CB 2679d-GT vector did not develop any anti-FIX antibody response and overall there was no statistically significant difference in immunogenicity compared with the cohorts injected with the R338L-Padua vector (Figure 2C-D). However, a single animal (of 26 total) injected with CB 2679d-GT developed transient anti-FIX antibodies (Figure 2D; supplemental Figure 4C). This rare event may potentially be related to some vector impurities (supplemental Figure 2). Most importantly, hemophilia B mice injected with the CB 2679d-GT or R338L-Padua vectors were protected from developing anti-FIX antibodies upon immune challenge with FIX protein and adjuvant, in contrast to control mice that did not undergo gene therapy (supplemental Figure 4A-B). This low immunogenicity of CB 2679d-GT is consistent with a comprehensive in silico and in vitro immunogenicity risk assessment of the corresponding purified FIX protein dalcinonacog alfa that showed no increased risk compared with the wild-type FIX.25 This was based on putative T-cell epitope content prediction (EpiMatrix) and in vitro dendritic cell/T-cell assays.
There was no difference in gene copy number and FIX messenger RNA levels between CB 2679d-GT and R338L-Padua vectors (Figure 2E-H; supplemental Figure 3A-B). Expression was exclusively restricted to the liver by virtue of the liver-specific AAT promoter (Figure 2G-H). Aspartate aminotransferase and alanine transaminase levels were within normal parameters after injecting the FIX-deficient mice with the CB 2679d-GT or R338L-Padua vectors (supplemental Figure 5A-D). This is consistent with normal liver histology without any discernable pathological immune infiltration or depositions (supplemental Figure 5E-H).
Although dalcinonacog alfa shows increased resistance to antithrombin (AT) inhibition,17 the current study revealed no increased thrombogenic risk after gene therapy with CB 2679d-GT. This is consistent with the absence of pathological elevation of D-dimer levels in vector-treated mice (supplemental Figure 6). Furthermore, in 2 clinical trials of dalcinonacog alfa, D-dimer, prothrombin F1+2, fibrinogen, and thrombin-antithrombin complex levels were not significantly affected.18,19 Although its inactivation by AT is ∼21-fold reduced compared with that for wild-type FIX, this FIX variant is still susceptible to AT inhibition albeit at a slower rate.
Collectively, these data support the efficacy and safety of CB 2679d-GT as a promising novel alternative to R338L-Padua for hemophilia B gene therapy.
For original data, please contact Thierry VandenDriessche at thierry.vandendriessche@vub.be.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Arun Srivastava for the self-complementary AAV backbone, and Mark Kay for the hemophilia B mice.
T.V. and M.K.C. were supported by the Fonds Wetenschappelijk Onderzoek–Vlaanderen (FWO) Research Foundation and a Vrije Universiteit Brussel (VUB) Industrieel Onderzoeksfonds Groups of Expertise in Applied Research (IOF-GEAR) grant. N.N. was supported by an FWO fellowship. The visual abstract was created with BioRender.com.
Authorship
Contribution: N.N. and D.D.W. designed and performed experiments, analyzed data, and wrote the paper; P.A.N., Q.H.P., and E.S.-K. performed experiments and analyzed data; M.K.C. and T.V. designed experiments, coordinated the work, analyzed data, and wrote the paper; and G.E.B. and J.L. designed experiments and wrote the paper.
Conflict-of-interest disclosure: G.E.B. and J.L. are employees and shareholders of Catalyst Biosciences, Inc. T.V. and M.K.C. received grant/research support from Catalyst Biosciences, Inc. The remaining authors declare no competing financial interests.
Correspondence: Thierry VandenDriessche, Department of Gene Therapy and Regenerative Medicine, Faculty of Medicine and Pharmacy, Vrije Universiteit Brussel, Building D, Room D365, Laarbeeklaan 103, B-1090 Brussels, Belgium; e-mail: thierry.vandendriessche@vub.be; and Marinee K. Chuah, Department of Gene Therapy and Regenerative Medicine, Faculty of Medicine and Pharmacy, Vrije Universiteit Brussel, Building D, Room D365, Laarbeeklaan 103, B-1090 Brussels, Belgium; e-mail: marinee.chuah@vub.be.
REFERENCES
Author notes
N.N. and D.D.W. are joint first authors.
M.K.C. and T.V. are joint last senior authors.