Key Points
Transfusing ABO-incompatible platelets may result in worse platelet recovery and outcomes in patients with ICH.
ABO compatibility should be considered for platelet transfusion approaches for patients with ICH.
Abstract
Acute platelet transfusion after intracerebral hemorrhage (ICH) given in efforts to reverse antiplatelet medication effects and prevent ongoing bleeding does not appear to improve outcome and may be associated with harm. Although the underlying mechanisms are unclear, the influence of ABO-incompatible platelet transfusions on ICH outcomes has not been investigated. We hypothesized that patients with ICH who receive ABO-incompatible platelet transfusions would have worse platelet recovery (using absolute count increment [ACI]) and neurological outcomes (mortality and poor modified Rankin Scale [mRS 4-6]) than those receiving ABO-compatible transfusions. In a single-center cohort of consecutively admitted patients with ICH, we identified 125 patients receiving acute platelet transfusions, of whom 47 (38%) received an ABO-incompatible transfusion. Using quantile regression, we identified an association of ABO-incompatible platelet transfusion with lower platelet recovery (ACI, 2 × 103cells per μL vs 15 × 103cells per μL; adjusted coefficient β, −19; 95% confidence interval [CI], −35.55 to −4.44; P = .01). ABO-incompatible platelet transfusion was also associated with increased odds of mortality (adjusted odds ratio [OR], 2.59; 95% CI, 1.00-6.73; P = .05) and poor mRS (adjusted OR, 3.61; 95% CI, 0.97-13.42; P = .06); however, these estimates were imprecise. Together, these findings suggest the importance of ABO compatibility for platelet transfusions for ICH, but further investigation into the mechanism(s) underlying these observations is required.
Introduction
Ongoing bleeding, or hematoma expansion, occurs early after intracerebral hemorrhage (ICH)1 and is the greatest driver of worse outcomes in these patients.2,3 Platelet transfusions had previously been implemented in hematoma expansion clinical treatment paradigms,4 particularly in patients with platelet dysfunction in efforts to improve outcomes. However, the Platelet Transfusion in Spontaneous Intracerebral Hemorrhage on Antiplatelet Agents (PATCH) trial revealed that platelet transfusions to “reverse” antiplatelet medication effects were not beneficial and could be harmful.5 The mechanisms underlying these findings are unclear but do not appear to be related to platelet preparation method or shelf life.6 It is also uncertain whether ABO incompatibility plays a role.
Providing ABO-compatible platelet transfusions is not the universal standard practice,7 even though platelets carry ABO antigens.8,9 This is driven by supply limitations and historical safety data of ABO-incompatible platelet transfusions10,11 However, data from patients without ICH suggests that incompatible transfusions are associated with poor platelet recovery12,13 and increased morbidity.14-16 Therefore, we hypothesized that patients with ICH receiving ABO-incompatible platelet transfusions would similarly have worse platelet recovery and outcomes than those receiving ABO-compatible transfusions. We also explored the relationship of ABO-incompatible platelet transfusions with hematoma expansion.
Study design
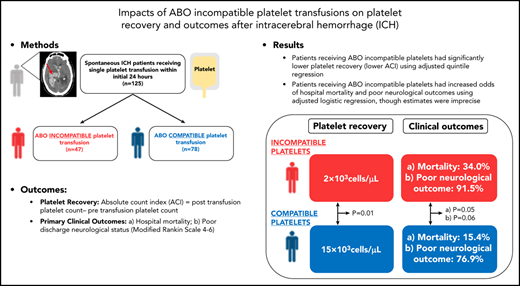
Consecutive spontaneous patients with ICH admitted to Columbia University Irving Medical Center were prospectively enrolled in an institutional review board–approved study. Baseline demographics, ICH characteristics, laboratory results, interventions, and outcomes were collected. Consent was provided by the patient or family. Patients enrolled between 2009 and 2016 and receiving a single platelet transfusion within the first 24 hours were studied. Patients transfused after 24 hours and those with secondary ICH, primary intraventricular hemorrhage, preceding anticoagulant use, or laboratory evidence of baseline coagulopathy17 were excluded (Figure 1).
Patients were managed according to American Heart Association guidelines4 with ICH treatment protocols described previously.18 ABO blood typing and complete blood count were obtained for all patients with ICH on admission. Prior to the PATCH trial (2016), platelets were acutely administered for patients with ICH who had suspected qualitative platelet dysfunction, but they were not always ABO compatible. Per center practice, platelet unit administration is performed by the blood bank with ABO-compatible platelets prioritized to be administered when available but ABO-incompatible platelets administered when compatible units were unavailable. Each platelet unit was apheresis collected and in plasma and met the American Association of Blood Banks standards (>3.0 × 1011 platelets in 90% of single units tested). Donor ABO type was obtained from the blood bank database, and platelet transfusion exposure was dichotomized as ABO-incompatible (major mismatch) vs ABO-compatible (identical or minor mismatch), as per prior studies.13 Platelet recovery was quantified using absolute count increments (ACIs).13
Clinical outcomes included mortality and poor neurological outcome defined as modified Rankin Scale (mRS) 4 to 6 at discharge and 3-month follow-up. Discharge outcomes were obtained by chart review and 3-month follow-ups via standardized phone interviews by trained research staff.18 Semiautomatic hematoma size measurements (Medical Imaging Processing, Analysis and Visualization, National Institutes of Health) were obtained for all head computed tomograms (CTs),18,19 and hematoma expansion between baseline and the 24-hour follow-up CT was calculated. Hematoma expansion was defined as a binary outcome using traditionally used thresholds (≥33% and/or ≥6 mL absolute growth)3,20 and as a continuous outcome variable of relative percent growth.
Intergroup differences (ABO-incompatible vs ABO-compatible platelet transfusion) were determined using analysis of variance or Mann-Whitney U tests for continuous variables and χ2 for categorical variables. Relationships between ABO-incompatible platelet transfusions and ACI were assessed using quantile regression, adjusting for transfused platelet age. We used these nonparametric methods to account for nonlinearity of variables in addition to accounting for all negative and positive values of ACI. Relationships between ABO-incompatible platelet transfusions and outcomes were assessed using logistic regression, adjusting for ICH score, a validated marker of ICH severity.21 Exploratory analyses to assess the relationship of platelet transfusion compatibility and hematoma expansion were performed using regression analyses after adjusting for relevant confounders.22 Statistical significance was set at P < .05. Analyses were performed using SPSS (v27; IBM).
Results and discussion
Of 125 patients with ICH meeting criteria for analyses (supplemental Figure 1, available on the Blood Web site), 38% (n = 47) received an ABO-incompatible platelet transfusion. There were no intergroup differences in demographics, ICH characteristics/severity, or age of transfused platelets (Table 1). Lower ACI (poorer platelet recovery) was seen in patients receiving ABO-incompatible platelet transfusions (median [IQR], 2 × 103cells per μL [−19 to 28] vs 15 × 103cells per μL [1-36]). We continued to identify an association between ABO-incompatible transfusion exposure with lower ACI after adjusting for platelet transfusion age (adjusted coefficient β, −19; 95% confidence interval [CI], − 35.55 to −4.44; P = .01). Given the possibility that differences between the time of transfusion and measurement of the posttransfusion platelet count between groups could result in differences in platelet recovery, we performed additional sensitivity analyses adjusting for this time covariate. In these analyses, we did not identify a change in the relationship of ABO-incompatible platelet transfusion exposure with lower ACI (adjusted coefficient β, −24; 95% CI, −38.62 to −10.59; P = .001). Additionally, patients receiving ABO-incompatible platelet transfusions had more frequent in-hospital mortality (34% vs 15%) and poor discharge mRS (91% vs 77%). There continued to be an association of ABO-incompatible platelet transfusion exposure with increased odds of hospital mortality (adjusted odds ratio [AOR], 2.59; 95% CI, 1.00-6.73; P = .05) and poor mRS at discharge (AOR, 3.61; 95% CI, 0.97-13.42; P = .06) after adjusting for ICH severity; however, these estimates were imprecise. No associations were seen for 3-month outcomes (Table 2). Because patients receiving ABO-incompatible platelet transfusions were more likely to have type O blood, sensitivity analyses were performed adjusting for blood type (O vs non-O) as a covariate to adjust for this potential confounder. In these analyses, there was no change in the relationship of incompatible platelet transfusion exposure on discharge mortality when incorporating patient blood type into the model (AOR, 1.96; 95% CI, 1.11-14.82; P = .04). Additional analyses stratified by blood type (O vs non-O) were limited due to sample size, but there were no notable differences in the relationship of incompatible platelet transfusion on discharge mortality among blood types (data not shown).
Intergroup characteristic differences between ICH patients receiving ABO-incompatible and compatible platelet transfusions
| . | All patients (N = 125) . | ABO incompatible (n = 47) . | ABO compatible (n = 78) . |
|---|---|---|---|
| Age, mean (SD), y | 65 (15.4) | 64 (16.1) | 65 (15.1) |
| Male, n (%) | 72 (57.6) | 26 (55.3) | 46 (59.0) |
| Race, n (%) | |||
| White | 29 (23.2) | 9 (19.1) | 20 (25.6) |
| Black | 35 (28.0) | 16 (34.0) | 19 (24.4) |
| Hispanic | 51 (40.8) | 19 (40.4) | 32 (41.0) |
| Other/unknown | 10 (8.0) | 3 (6.4) | 7 (9.0) |
| Medical history, n (%) | |||
| Atrial fibrillation | 5 (4.0) | 1 (2.1) | 4 (5.1) |
| Coronary artery disease | 19 (15.2) | 7 (14.9) | 12 (15.4) |
| Dyslipidemia | 34 (27.2) | 11 (23.4) | 23 (29.5) |
| Hypertension | 97 (76.0) | 38 (80.9) | 57 (73.1) |
| Diabetes | 32 (25.6) | 10 (21.3) | 22 (28.2) |
| Clinical/radiographic | |||
| ICH score, median (IQR) | 2 (1-3) | 2 (1-3) | 1.5 (1-3) |
| ICH volume, median (IQR), mL | 13 (5-33) | 14 (3-41) | 12 (5-28) |
| Presence of IVH, n (%) | 66 (52.8) | 25 (53.2) | 41 (52.6) |
| ICH location, n (%) | |||
| Lobar | 36 (28.8) | 10 (21.3) | 26 (33.3) |
| Deep | 80 (64.0) | 35 (74.5) | 45 (57.7) |
| Infratentorial | 6 (4.8) | 2 (4.3) | 4 (5.1) |
| Brainstem | 3 (2.4) | 0 (0.0) | 3 (3.8) |
| Platelet age, median (IQR), d | 4 (4-5) | 4 (3-5) | 4 (4-5) |
| Time to transfusion, median (IQR), h | 3 (2-7) | 3 (2-7) | 4 (2-7) |
| Patient blood type, n (%) | |||
| O | 66 (52.8) | 41 (87.2) | 25 (32.1) |
| A | 42 (33.6) | 2 (4.3) | 40 (51.3) |
| B | 13 (10.4) | 4 (8.5) | 9 (11.5) |
| AB | 4 (3.2) | 0 (0.0) | 4 (5.1) |
| Mismatch type, n (%) | |||
| None | 63 (50.4) | N/A | 63 (80.8) |
| Major | 41 (32.8) | 41 (87.2) | N/A |
| Minor | 15 (12.0) | N/A | 15 (19.2) |
| Both | 6 (4.8) | 6 (12.8) | N/A |
| . | All patients (N = 125) . | ABO incompatible (n = 47) . | ABO compatible (n = 78) . |
|---|---|---|---|
| Age, mean (SD), y | 65 (15.4) | 64 (16.1) | 65 (15.1) |
| Male, n (%) | 72 (57.6) | 26 (55.3) | 46 (59.0) |
| Race, n (%) | |||
| White | 29 (23.2) | 9 (19.1) | 20 (25.6) |
| Black | 35 (28.0) | 16 (34.0) | 19 (24.4) |
| Hispanic | 51 (40.8) | 19 (40.4) | 32 (41.0) |
| Other/unknown | 10 (8.0) | 3 (6.4) | 7 (9.0) |
| Medical history, n (%) | |||
| Atrial fibrillation | 5 (4.0) | 1 (2.1) | 4 (5.1) |
| Coronary artery disease | 19 (15.2) | 7 (14.9) | 12 (15.4) |
| Dyslipidemia | 34 (27.2) | 11 (23.4) | 23 (29.5) |
| Hypertension | 97 (76.0) | 38 (80.9) | 57 (73.1) |
| Diabetes | 32 (25.6) | 10 (21.3) | 22 (28.2) |
| Clinical/radiographic | |||
| ICH score, median (IQR) | 2 (1-3) | 2 (1-3) | 1.5 (1-3) |
| ICH volume, median (IQR), mL | 13 (5-33) | 14 (3-41) | 12 (5-28) |
| Presence of IVH, n (%) | 66 (52.8) | 25 (53.2) | 41 (52.6) |
| ICH location, n (%) | |||
| Lobar | 36 (28.8) | 10 (21.3) | 26 (33.3) |
| Deep | 80 (64.0) | 35 (74.5) | 45 (57.7) |
| Infratentorial | 6 (4.8) | 2 (4.3) | 4 (5.1) |
| Brainstem | 3 (2.4) | 0 (0.0) | 3 (3.8) |
| Platelet age, median (IQR), d | 4 (4-5) | 4 (3-5) | 4 (4-5) |
| Time to transfusion, median (IQR), h | 3 (2-7) | 3 (2-7) | 4 (2-7) |
| Patient blood type, n (%) | |||
| O | 66 (52.8) | 41 (87.2) | 25 (32.1) |
| A | 42 (33.6) | 2 (4.3) | 40 (51.3) |
| B | 13 (10.4) | 4 (8.5) | 9 (11.5) |
| AB | 4 (3.2) | 0 (0.0) | 4 (5.1) |
| Mismatch type, n (%) | |||
| None | 63 (50.4) | N/A | 63 (80.8) |
| Major | 41 (32.8) | 41 (87.2) | N/A |
| Minor | 15 (12.0) | N/A | 15 (19.2) |
| Both | 6 (4.8) | 6 (12.8) | N/A |
IQR, interquartile range; IVH, intraventricular hemorrhage; N/A, not applicable; SD, standard deviation.
Relationship of ABO-incompatible platelet transfusion on platelet recovery and poor neurological outcomes
| Platelet recovery . | ABO incompatible . | ABO compatible . | Adjusted β (95% CI)* . | AOR (95% CI)† . | P . |
|---|---|---|---|---|---|
| ACI (platelets, ×103/μL),‡ median (IQR) | 2 (−19 to 28) | 15 (1-36) | −19.00 (−35.55 to −4.44) | — | .01 |
| Neurologic outcome, n (%) | |||||
| Discharge death | 16 (34.0) | 12 (15.4) | — | 2.59 (1.00-6.73) | .05 |
| Discharge poor mRS (4-6) | 43 (91.5) | 60 (76.9) | — | 3.61 (0.97-13.42) | .06 |
| 3-mo poor mRS (4-6)§ | 30 (75.0) | 34 (69.4) | — | 1.47 (0.52-4.14) | .47 |
| Platelet recovery . | ABO incompatible . | ABO compatible . | Adjusted β (95% CI)* . | AOR (95% CI)† . | P . |
|---|---|---|---|---|---|
| ACI (platelets, ×103/μL),‡ median (IQR) | 2 (−19 to 28) | 15 (1-36) | −19.00 (−35.55 to −4.44) | — | .01 |
| Neurologic outcome, n (%) | |||||
| Discharge death | 16 (34.0) | 12 (15.4) | — | 2.59 (1.00-6.73) | .05 |
| Discharge poor mRS (4-6) | 43 (91.5) | 60 (76.9) | — | 3.61 (0.97-13.42) | .06 |
| 3-mo poor mRS (4-6)§ | 30 (75.0) | 34 (69.4) | — | 1.47 (0.52-4.14) | .47 |
Platelet recovery model adjusted for age of transfused platelets.
Neurologic outcome model adjusted for ICH score.
ACI scores were missing for 2.4% (n = 3); percentages are from 122 patients, 76 with compatible transfusions and 46 with incompatible transfusions.
Three-month loss to follow-up was 28.8% (n = 36); percentages are from 89 patients, 49 with compatible transfusions and 40 with incompatible transfusions.
To assess whether worse platelet recovery in patients receiving ABO-incompatible platelet transfusions could impact hemostasis, we performed a subgroup analysis investigating the relationship between ABO-incompatible platelet transfusion exposure on hematoma expansion in those with available neuroimaging data (n = 111). There were no significant intergroup differences in hematoma expansion defined as a binary outcome variable (25% vs 20%). When defined as a continuous variable of relative growth, we identified larger hematoma expansion in patients receiving ABO-incompatible platelet transfusions (4.4% vs −0.9%), but this was not statistically significant (adjusted coefficient β, −0.08; 95% CI, −37.85 to 14.75; P = .39) (supplemental Table 1).
These findings suggest that ABO-incompatible platelet transfusions are associated with poor platelet recovery and worse in-hospital outcomes. Although the PATCH trial results appropriately led many centers to rethink the role of platelet transfusions for acute coagulopathy reversal in patients with ICH, it is unclear whether the harmful effects seen with platelet transfusions in this study were partly explained by transfusing ABO-incompatible platelets.
Although it could be speculated that the transfusion of ABO-incompatible platelets leads to impaired platelet recovery, more hematoma expansion, and worse outcomes, it is important to note that significant differences in hematoma expansion differences were not identified between patients receiving ABO-incompatible vs ABO-compatible platelet transfusions in our cohort. It is unclear whether the small sample size of our cohort prevented identifying significant intergroup differences in hematoma expansion. However, the deleterious relationship of ABO-incompatible transfusions on poor outcome was quite large despite the aforementioned sample size limitations, without any obvious evidence of disease severity driving these differences. It is feasible that the relationship between ABO-incompatible transfusions and poor outcome was mediated by other unmeasured confounders such as postexposure infections or thromboembolic events, which have known associations with poor ICH outcomes.23,24 Separate literature has identified that the transfusion of ABO-incompatible platelets can lead to the formation of immune complexes and impaired platelet function.25 This could provide driving mechanisms for downstream complications related to thromboinflammation (ie, infections and/or thromboembolic events). Furthermore, it is speculative, yet possible, that these immune complexes can also lead to accelerated platelet clearance, leading to the negative changes in ACI seen more often in our incompatible platelet transfusion group. The negative ACI values seen in our study may also reflect consumption in developing clots and the known variation of the platelet cell count assay itself.26,27
It could also be hypothesized that underlying patient blood type, rather than the incompatible platelet transfusion itself, could be a driver for the outcome differences seen in our cohort. We expectedly identified that patients with ICH receiving incompatible platelet transfusions were more likely to have type O blood. There are known differences in von Willebrand factor levels between patients with type O vs non-O blood,28 and it could be posited that this factor, or inherent differences in clinical ICH outcomes among patients with different blood types, played a role in our intergroup differences. However, we performed additional analyses accounting for underlying blood type and continued to identify a relationship between incompatible platelet transfusion exposure and poor outcomes. These findings in conjunction with prior literature, which has not identified relationships of patient ABO blood type and ICH outcomes,29,30 suggest that future investigations should focus on the impact of incompatible platelet transfusions themselves on outcomes.
While our study’s strengths include prospective data collection, quantitative neuroimaging analyses, multidisciplinary ICH characteristics/outcome adjudication, and uniform treatment protocol implementation, there are several limitations worth mentioning. Our small, single-center cohort sample size not only necessitates external replication but also prohibited a closer look at the impacts of both major and minor incompatibility on outcomes. Though we used similar approaches as prior studies (grouping minor and major mismatch together), prior studies have identified hemostasis and mortality differences in patients receiving minor incompatible platelet transfusion products.31 There may very well be differential impacts on outcomes between these 2 groups that would be clarified with a larger dataset. Additionally, the exclusion of patients receiving multiple transfusions or transfusions after 24 hours limited the generalizability of our findings. However, our analyses assessing relationships between transfusion and hematoma expansion necessitated these exclusions, as this complication primarily occurs in the first 24 hours.32 Further limitations included the inability to use corrected count increment due to lack of body surface area data and the potential presence of unmeasured confounders, such as the absence of cerebral edema measurements, infection, or thrombotic complications, to evaluate other drivers behind the relationship between ABO-incompatible platelet transfusions and outcomes. Additionally, the absence of specific platelet unit data or donor data prohibited a closer look at whether individual platelet count variability in the specific unit or variable expression of ABO antigens and specific mismatches, respectively, played a role in our outcomes. Lastly, the utilization of ACI may not provide an accurate assessment of physiologic coagulation changes after platelet transfusion as opposed to other specific laboratory assays of platelet activity or hemostatic assays.
Given these limitations, further investigation is required to replicate and investigate the mechanisms behind our findings. However, at the very least, our findings appear to be consistent with analogous studies in non-ICH patient populations showing that ABO-incompatible platelet transfusions are associated with poor platelet recovery10,13,16 and increased morbidity.14-16 Additional prospective studies will be required to assess whether ABO-identical platelet transfusions are indicated in this vulnerable patient population.
For original data, please contact the corresponding author.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank the patients, nurses, and study coordinators that made this study possible.
D.R. receives funding support from the National Blood Foundation.
Authorship
Contribution: J.M.-B. performed research, analyzed data, and drafted the manuscript; C.B.B. and F.C.-P. performed research and edited the manuscript; A.B. assisted with data analysis and edited the manuscript; E.A.H. and E.S.C. designed research, performed research, and edited the manuscript; R.O.F. designed research and edited the manuscript; M.S.V.E. edited the manuscript; S.A., S.P., and J.C. performed research and edited the manuscript; and D.R. designed research and overview of the study design, performed research, analyzed data, and drafted the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: David Roh, New York-Presbyterian/Columbia University Irving Medical Center, 177 Fort Washington Ave, Milstein Hospital 8GS-300, New York, NY 10032; e-mail: dr2753@cumc.columbia.edu.

Comments
Mechanisms of impaired hemostasis after ABO mismatched platelet transfusions