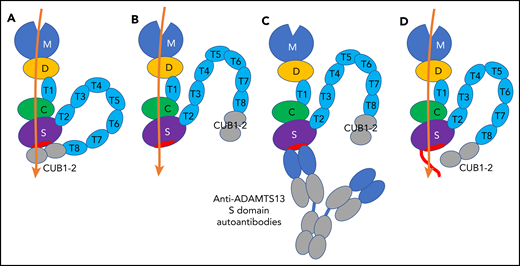
Schematic representation of 2 conformations of ADAMTS13, ADAMTS13-autoantibody complex, and NGLY3-ADAMTS13 variant. (A) Closed conformation of ADAMTS13. The closed conformation is maintained via an interaction of the S domain with the C-terminal CUB domains that diminishes its proteolytic activity. (B) Open conformation of ADAMTS13. The exosites in the D, C, and S domains bind to the unfolded A2 polypeptide chain (orange), and the binding enhances the proteolytic function of the M domain. (C) Binding of anti-ADAMTS13 S domain autoantibodies to the S domain. The binding interferes with the proteolytic function. (D) NGLY3-ADAMTS13 variant. The variant has an N-glycan at position 608 (red line) that interferes with the binding of anti-ADAMTS13 S domain autoantibodies, but not the binding of the unfolded A2 polypeptide chain. The conformational state of the variant is uncertain.
Schematic representation of 2 conformations of ADAMTS13, ADAMTS13-autoantibody complex, and NGLY3-ADAMTS13 variant. (A) Closed conformation of ADAMTS13. The closed conformation is maintained via an interaction of the S domain with the C-terminal CUB domains that diminishes its proteolytic activity. (B) Open conformation of ADAMTS13. The exosites in the D, C, and S domains bind to the unfolded A2 polypeptide chain (orange), and the binding enhances the proteolytic function of the M domain. (C) Binding of anti-ADAMTS13 S domain autoantibodies to the S domain. The binding interferes with the proteolytic function. (D) NGLY3-ADAMTS13 variant. The variant has an N-glycan at position 608 (red line) that interferes with the binding of anti-ADAMTS13 S domain autoantibodies, but not the binding of the unfolded A2 polypeptide chain. The conformational state of the variant is uncertain.
In this issue of Blood, Ercig et al report that ADAMTS13 modified by N-glycosylation functions even in the presence of sera obtained from patients with immune-mediated thrombotic thrombocytopenic purpura (iTTP).1 This variant may be promising for treatment of iTTP patients.
Thrombotic thrombocytopenic purpura (TTP) is a life-threatening disease with microvascular occlusion characterized by systemic platelet aggregation, multiorgan ischemia, thrombocytopenia, and fragmentation of red blood cells.2-4 There are 2 types of TTP, congenital TTP characterized by either homozygous or compound heterozygous mutations in the ADAMTS13 gene, and iTTP characterized by anti-ADAMTS13 autoantibodies. ADAMTS13 is a von Willebrand factor (VWF)-cleaving protease that consists of discrete domains, that is, a metalloproteinase domain (M), a disintegrin-like domain (D), a first thrombospondin type 1 repeat (T1), a cysteine-rich domain (C), a spacer domain (S), 7 thrombospondin type 1 repeats (T2 to T8), and 2 CUB domains (CUB1 to CUB2) (see figure).2-4 VWF is secreted from endothelial cells as highly prothrombotic ultralarge multimers. The shear force in blood circulation stretches VWF multimers and unfolds the VWF A2 domain.2 ADAMTS13 cleaves the exposed Tyr1605-Met1606 bond in this domain and reduces the VWF multimer size to avoid spontaneous platelet binding.
Under normal conditions, ADAMTS13 holds a closed conformation maintained by the interaction between the S domain and C-terminal CUB domains (see figure panel A). Once the VWF D4-CK domains bind to the ADAMTS13 CUB domains, a conformational change occurs on ADAMTS13 from a closed to an open state, and the S domain exosite, composed of residues Arg568, Phe592, Arg660, Tyr661, and Tyr665 (see figure panel B, red ellipse),5 is exposed and binds to the unfolded VWF A2 domain.4,6,7 Binding of anti-ADAMTS13 autoantibodies against the CUB domains also induces the same conformational change.4 As a consequence, ADAMTS13 binds to the unfolded VWF A2 domain through the exosites on the D, C, and S domains, resulting in a conformational change in the M domain to facilitate the scissile bond cleavage.7,8 Thus, the multiple subsite interactions between the ADAMTS13 exosites and the unfolded VWF A2 domain are very important for the VWF multimer size reduction by ADAMTS13. If these interactions are interrupted with, for example, anti-ADAMTS13 inhibitory autoantibodies, the VWF multimers are accumulated in plasma, leading to platelet-rich microthrombi.
Very interestingly, most anti-ADAMTS13 inhibitory autoantibodies in patients with iTTP are directed toward the S domain (see figure panel C),9 although in many patients, anti-ADAMTS13 autoantibodies with epitopes in other ADAMTS13 domains are also present. Anti-ADAMTS13 S domain autoantibodies likely interfere with the ADAMTS13 binding to the unfolded VWF A2 domain, resulting in strong inhibition of the ADAMTS13 activity. An ADAMTS13 S domain exosite variant with 5 amino acid substitutions, Arg568Lys, Phe592Tyr, Arg660Lys, Tyr661Phe, and Tyr665Phe, showed resistance against the inhibitory function of autoantibodies from iTTP patients and expressed even higher proteolytic activity (a so-called gain-of-function variant).10 For therapeutic use of ADAMTS13, the inhibition of the ADAMTS13 activity by autoantibodies is undesired, and the autoantibody-resistant ADAMTS13 variants are beneficial and promising.
Ercig et al undertook an elegant and sophisticated strategy, using prediction guided by structural bioinformatics to produce novel ADAMTS13 variants resistant to anti-ADAMTS13 S domain autoantibodies. First, they generated docking models of the ADAMTS13 C-S domains and the variable fragments from patient-derived monoclonal anti-ADAMTS13 autoantibodies. This enabled them to identify a larger epitope, consisting of 11 amino acid residues, than previously identified for autoantibodies on the S domain. Then, they produced novel single Ala ADAMTS13 variants based on the new models. Unfortunately, all of the Ala variants failed to escape autoantibody bindings. They further produced 6 ADAMTS13 variants containing the N-glycan attachment sequences based on the models, and finally, identified 1 N-glycosylated variant (NGLY3-ADAMTS13 with a p.Lys608Asn substitution) that showed autoantibody-resistant properties.
The NGLY3-ADAMTS13 variant thus identified showed low binding (<50%) against the sera in 10 out of 13 iTTP patients. The NGLY3 variant showed activity similar to the wild-type ADAMTS13 using the peptidyl VWF substrate as well as the VWF multimer under flow conditions. The variant retained the peptidyl VWF substrate-cleaving activity in the presence of an excess of patient autoantibodies, indicating that it had an autoantibody-resistant property. The presence of N-glycan on the variant was confirmed using the mass spectrometry analysis. Based on these findings, they concluded that a newly introduced N-glycan on Asn608 in ADAMTS13 hindered the autoantibody binding to the variant that conferred the autoantibody resistance (see figure panel D). They proposed this novel variant as a therapeutic option for treatment of iTTP.
There are several caveats to be considered. As the NGLY3-ADAMTS13 variant is engineered in the central S domain, it can bind to the autoantibodies against the C-terminal domains. For treatment of iTTP patients, studies on the risk for immunization against the NGLY3 variant will be needed.
Ercig et al showed that an approach using bioinformatics-guided prediction is valid and important for producing autoantibody-resistant ADAMTS13 variants that advance the field of iTTP. The predictions of the introduced N-glycan sites on the S domain should be particularly stimulating and informative for researchers. The concept that artificially inserted N-glycans interfere with the binding of inhibitory autoantibodies while retaining normal levels of proteolytic activity is also valuable. The study by Ercig et al has high therapeutic potential and is a welcome advance in the field.
Conflict-of-interest disclosure: The author is a member of the Clinical Advisory Board for Takeda and received a speaker fee from Shino-Test. He has a patent for specific substrate and activity measurement method for ADAMTS13 issued in Japan and the United States.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal