TO THE EDITOR:
Recipients of solid organ transplant are at increased risk to develop posttransplant lymphoproliferative disorder (PTLD).1 There are no uniform guidelines for treatment of PTLD; strategies include withdrawal of immunosuppression, single-agent rituximab, and, for aggressive PTLD, the standard is chemoimmunotherapy. However, only 30% to 40% achieve a durable response.2,3 Chimeric antigen receptor (CAR) T-cell therapy is a novel treatment with a substantial response (52% to 82%) in patients with relapsed/refractory diffuse large B-cell lymphoma (r/r DLBCL),4,5 but its safety and efficacy in PTLD is unknown. Two CAR T-cell therapy products, axi-cel and tisagenlecleucel, are currently approved for r/r DLBCL and both are derived from autologous T cells. Because solid organ transplant recipients are on long-term immunosuppressive therapy to prevent allograft rejection, there is concern about the feasibility of generating an effective autologous CAR T-cell product. Here, we report the safety and efficacy of autologous CAR T-cell therapy in 3 patients with r/r DLBCL and kidney allograft who were treated with axi-cel in 2019 to 2020.
This study was approved by the institutional review board of the MD Anderson Cancer Center and conducted in accordance with the Declaration of Helsinki. Toxicities related to axi-cel including cytokine release syndrome (CRS) and immune effector cell-associated neurotoxicity syndrome (ICANS) were graded according to the consensus American Society of Transplantation and Cellular Therapy grading system,6 and disease response was assessed per the 2014 Lugano classification.7 Axi-cel was administered as per published protocol.4 Axi-cel expansion and persistence were estimated by quantitative polymerase chain reaction (qPCR) of the integrated genome of the retrovirus encoding axi-cel in available peripheral blood samples during the first 30 days after axi-cel in patient 1 and day +106 in patient 3.8 Peripheral blood B-cell numbers were determined by flow cytometry and used as surrogate measures of functional CAR-T persistence. Donor-derived cell-free DNA (dd-cfDNA) originating from graft cells undergoing cell injury and death was monitored and quantified by measuring single nucleotide polymorphisms levels in recipient`s blood, which is a standard procedure at our center after immunosuppression is tapered or discontinued. A value of >1.0% dd-cfDNA was considered associated with active graft rejection with a negative predictive value of 84%.9
Patient 1 was a 38-year-old man who underwent kidney transplant for mesalamine-induced nephrotoxicity 10 years before diagnosis of stage IV germinal center B-cell (GCB) DLBCL, was Epstein-Barr virus (EBV)−, and had International Prognostic Index (IPI) score of 3. The disease was refractory to frontline therapy with rituximab, etoposide, prednisone, vincristine, cyclophosphamide, and doxorubicin (R-EPOCH), salvage therapy with gemcitabine, oxaliplatin, and rituximab and subsequently was eligible for axi-cel. Immunosuppressive therapy for kidney transplant consisted of mycophenolate mofetil that was discontinued at the time of DLBCL diagnosis, tacrolimus that was discontinued 2 weeks before leukapheresis, and prednisone that was continued at 5 mg per day. He developed grade 1 CRS on day +6 but did not develop ICANS. Disease assessment with positron emission tomography (PET) scan showed a complete response (CR) at week +4 that was sustained up to week +28 after axi-cel (Figure 1A). The patient remained off immunosuppressive therapy with grossly stable kidney function until week +21 when he had significant rise in serum creatinine. Notably, this was preceded by subclinical evidence of kidney allograft rejection diagnosed at week +16 by a rising dd-cfDNA level from 0.55% to 4.6% and confirmed with a kidney biopsy as borderline cell-mediated rejection (Figure 2). Furthermore, he subsequently developed donor-specific antibodies at week +28, suggesting concurrent antibody-mediated rejection and possibly the loss of functional CAR T-cell persistence because this was associated with a rise in immunoglobulin G (IgG) level (from 650 to 1474 mg/dL; reference range, 610-1616 mg/dL) and CD19 cell count (from 0 to 64 cells per μL) at week +4 after axi-cel and after the diagnosis of graft rejection, respectively. Adequate CAR T-cell expansion was observed within the first 30 days after infusion (Figure 3). However, blood samples were unavailable to assess persistence beyond day +30. His lymphoma remained in remission while renal function declined gradually. At this point, it might have been reasonable to restart the immunosuppression therapy to salvage the graft; however, taking into consideration the lack of prior data estimating the risk of disease relapse with restarting calcineurin inhibitors and weighing the refractory nature of his disease before response with axi-cel therapy, the treating team and patient had an extensive discussion, and the shared decision was to avoid restarting immunosuppressive therapy to minimize potential risk of disease relapse.
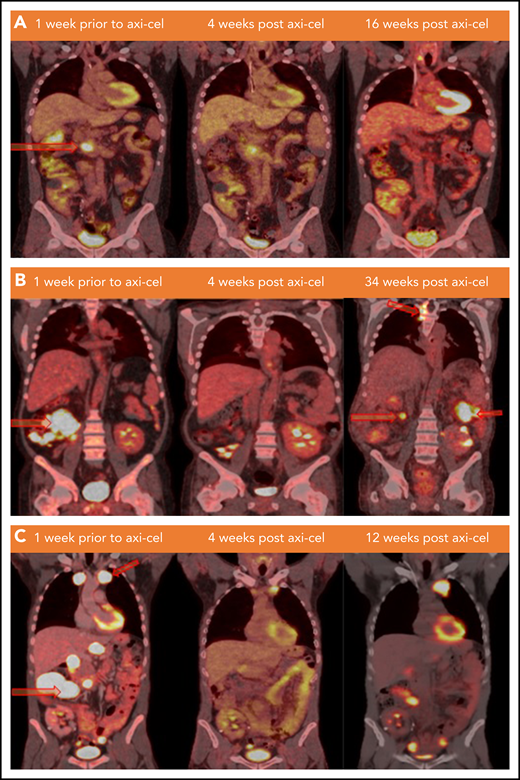
Lymphoma status before and after CAR T-cell infusion. (A) PET/CT scans of patient 1 at the specified time points. Hypermetabolic pancreatic lesion is indicated at week −1 (arrow). (B) PET/CT scans of patient 2 demonstrating from left to right: hypermetabolic cecal mass and ileocolic lymphadenopathy lesions (arrows), no FDG avid lesion (remission), and development of new lesions in thoracic spine, left posterior abdomen, and in the right mid-abdomen (relapse) at weeks −1, +4, and +34 of axi-cel therapy, respectively. (C) PET/CT scan of patient 3 demonstrating from left to right: hypermetabolic left anterior mediastinal mass and multiple abdominal lymph nodes (arrows), interval decreasing in size and metabolic activity of these lesions (partial response), and progression at weeks −1, +4, and +12 of axi-cel therapy, respectively. FDG, fluorodexoglucose.
Lymphoma status before and after CAR T-cell infusion. (A) PET/CT scans of patient 1 at the specified time points. Hypermetabolic pancreatic lesion is indicated at week −1 (arrow). (B) PET/CT scans of patient 2 demonstrating from left to right: hypermetabolic cecal mass and ileocolic lymphadenopathy lesions (arrows), no FDG avid lesion (remission), and development of new lesions in thoracic spine, left posterior abdomen, and in the right mid-abdomen (relapse) at weeks −1, +4, and +34 of axi-cel therapy, respectively. (C) PET/CT scan of patient 3 demonstrating from left to right: hypermetabolic left anterior mediastinal mass and multiple abdominal lymph nodes (arrows), interval decreasing in size and metabolic activity of these lesions (partial response), and progression at weeks −1, +4, and +12 of axi-cel therapy, respectively. FDG, fluorodexoglucose.
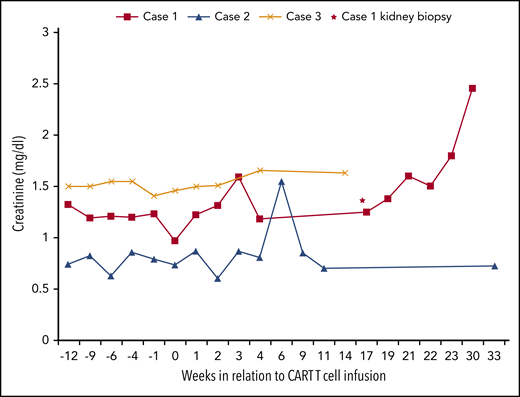
Serum creatinine levels in relation to CAR T-cell infusion. The horizontal axis represents time (weeks) in relation to CAR T-cell infusion, and the vertical axis represents serum creatinine value. Baseline serum creatinine in cases 1, 2, and 3 were 1.20, 0.74, and 1.53 mg/dL, respectively, at time of CAR T-cell infusion. Case 1 developed AKI at week 21 that was attributed to possible acute graft rejection (Banff classification; i1,t1 v0, g0, ptc 0, c4d0, cg0, mm0, ah2, cv1, ci 0, and ct0). His dd-cfDNA level increased from 0.55% to 4.6% at week 16 with no detectable DSA. Repeat DSA at week 28 was positive. Case 3 kidney function remained stable throughout the therapy, and case 2 developed AKI at week 7 that was attributed to hypovolemia and resolved with fluid resuscitation. ah, arteriolar hyalinosis; AKI, acute kidney injury; ci, interstitial fibrosis; ct, tubular atrophy; cv, vascular fibrous intima thickening; DSA, donor-specific antibodies; i, interstitial inflammation; g, glomerulitis; mm, mesangial matrix expansion; ptc, peritubular capillaritis; t, tubulitis; v, arteritis.
Serum creatinine levels in relation to CAR T-cell infusion. The horizontal axis represents time (weeks) in relation to CAR T-cell infusion, and the vertical axis represents serum creatinine value. Baseline serum creatinine in cases 1, 2, and 3 were 1.20, 0.74, and 1.53 mg/dL, respectively, at time of CAR T-cell infusion. Case 1 developed AKI at week 21 that was attributed to possible acute graft rejection (Banff classification; i1,t1 v0, g0, ptc 0, c4d0, cg0, mm0, ah2, cv1, ci 0, and ct0). His dd-cfDNA level increased from 0.55% to 4.6% at week 16 with no detectable DSA. Repeat DSA at week 28 was positive. Case 3 kidney function remained stable throughout the therapy, and case 2 developed AKI at week 7 that was attributed to hypovolemia and resolved with fluid resuscitation. ah, arteriolar hyalinosis; AKI, acute kidney injury; ci, interstitial fibrosis; ct, tubular atrophy; cv, vascular fibrous intima thickening; DSA, donor-specific antibodies; i, interstitial inflammation; g, glomerulitis; mm, mesangial matrix expansion; ptc, peritubular capillaritis; t, tubulitis; v, arteritis.
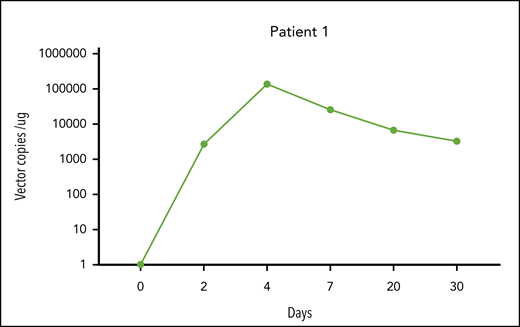
CAR T-cell expansion in peripheral blood in patient 1. The horizontal axis represents time (days) in relation to CAR T-cell infusion, and the vertical axis represents vector copies per microgram of buffy coat DNA. We quantified the integrated genome of the retrovirus encoding the anti-CD19 CAR (axi-cel) by quantitative PCR.8 DNA was extracted from buffy coat samples with DNeasy Blood and Tissue Kit (Qiagen) as per the manufacturer’s instructions. DNA was amplified with primers and probes (IDT Technologies) complementary to specific sequences within the axi-cel vector. The sequences of the primers and probes were as follows: forward primer, 5′ attcgccagcctccacgaaa 3′; reverse primer, 5′ ggtcagtctggatttgagagc 3′; probe, 5′ FAM gggtctggaZENgtggctgggagt3′IBFQ. The standard curve was established using serial dilutions of the known quantity of the DNA encoding the transgene. We performed amplifications using the C1000 Touch Thermal Cycler (Bio-Rad) Real-Time PCR System as per the manufacturer’s instructions.
CAR T-cell expansion in peripheral blood in patient 1. The horizontal axis represents time (days) in relation to CAR T-cell infusion, and the vertical axis represents vector copies per microgram of buffy coat DNA. We quantified the integrated genome of the retrovirus encoding the anti-CD19 CAR (axi-cel) by quantitative PCR.8 DNA was extracted from buffy coat samples with DNeasy Blood and Tissue Kit (Qiagen) as per the manufacturer’s instructions. DNA was amplified with primers and probes (IDT Technologies) complementary to specific sequences within the axi-cel vector. The sequences of the primers and probes were as follows: forward primer, 5′ attcgccagcctccacgaaa 3′; reverse primer, 5′ ggtcagtctggatttgagagc 3′; probe, 5′ FAM gggtctggaZENgtggctgggagt3′IBFQ. The standard curve was established using serial dilutions of the known quantity of the DNA encoding the transgene. We performed amplifications using the C1000 Touch Thermal Cycler (Bio-Rad) Real-Time PCR System as per the manufacturer’s instructions.
Patient 2 was a 44-year-old man who underwent kidney transplant for Alport syndrome 10 years before diagnosis of stage IV GCB-DLBCL, was EBV−, and had an IPI score of 3. The DLBCL was refractory to rituximab, cyclophosphamide, doxorubicin, prednisone, and vincristine after 2 cycles, at which point he was eligible to receive axi-cel on a clinical trial (#NCT03761056). Immunosuppressive therapy for kidney transplant consisted of sirolimus that was discontinued 4 weeks before leukapheresis and prednisone 30 mg per day that was reduced to 4.5 mg/day and then discontinued at weeks −4 and +1 of leukapheresis, respectively. He developed grade 1 CRS with fever on day +5 and grade 3 ICANS on day +8 that was treated with intravenous dexamethasone for 6 days with subsequent resolution by day +13. Six weeks after axi-cel infusion, the patient was hospitalized for treatment of nosocomial pneumonia, and he had acute kidney injury on the day of admission attributed to hypovolemia, which recovered with intravenous fluid within 24 hours. A PET scan on day +30 revealed complete response that was sustained for more than 12 weeks and was associated with hypogammaglobinemia and B-cell aplasia (IgG levels of 319, 247, and 399 mg/dL and undetectable periphral blood CD19+ cells at weeks +4, +8, and +12 of axi-cel infusion, respectively). However, his disease relapsed at +34 weeks, at which point he was treated with radiation plus hyperfractionated cyclophosphamide. CAR-T persistence, CD19+ cells, and IgG were not measured after relapse. Kidney function remained stable at week +36, with <1% dd-cfDNA. He remained off immunosuppressive medications except prednisone 5 mg/day, which was restarted as maintenance therapy at week 8.
Patient 3 was a 41-year-old man with a history of kidney transplant for treatment of granulomatous polyangiitis 7 years prior to diagnosis of stage IV GCB-DLBCL, was EBV−, and had an IPI of 2. The lymphoma was refractory to frontline therapy with R-EPOCH and multiple salvage regimens including rituximab, gemcitabine, dexamethasone, and cisplatin; rituximab, etoposide, methylprednisolone, cytarabine, and cisplatin; and polatuzumab vedotin, bendamustine, and rituximab. Immunosuppressive therapy for kidney transplant consisted of sirolimus that was discontinued 2.5 weeks before leukapheresis and prednisone that was continued at 5 mg/day. No CRS or ICANS was observed after axi-cel therapy. At week +4, a PET scan revealed partial response; however, he developed progressive disease at week +12 despite detectable CAR+ cells in the peripheral blood (4397 CAR copies per µg DNA at week 15) and ongoing B-cell aplasia (undetectable CD19+ cells at weeks +4 and +19 after axi-cel). His kidney function remained stable, and dd-cfDNA levels were <1%. PET-computed tomography scan results and serum creatinine values for patients 1 to 3 are summarized in Figures 1 and 2, respectively.
Given the T-cell inhibitory effects of immunosuppressive therapy, patients with monomorphic PTLD from solid organ transplant are excluded from trials, and there is a significant gap in our understanding of safety and efficacy of CAR T-cell therapy in this patient population. A recent published paper described a poor outcome associated with axi-cel use in treatment of 3 patients with r/r DLBCL after solid organ transplant: patient 1 with pancreas after kidney transplant, patient 2 with heart transplant, and patient 3 with kidney transplant. Immunosuppression was not suspended during the first 30 days after axi-cel therapy in the first 2 patients. Interestingly, all 3 patients developed toxicities and did not have disease response, and patient 3, who was off immunosuppression, had sepsis and died at day 15 of therapy.10
In our series and despite the discontinuation of immunosuppressive therapy in all patients before axi-cel infusion, 2 patients had disease relapse at 3 and 8 months, respectively, and with laboratory findings suggestive of axi-cel persistence at the time of relapse in 1 case. Additionally, 2 patients had no clinical signs of acute rejection while being off immunosuppressive therapy for 4 and 9 months, respectively. Recipients of kidney allograft in general have very low risk of developing acute rejection after 10 years of transplant; it accounts for ≤2% of graft loss etiology. However, in patients with PTLD after multiple years of kidney transplant and treated by discontinuation of calcineurin inhibitor and chemotherapy, acute rejection remains a concern and has a reported incidence as high as 12%. Notably, none of the reported acute rejections were reported in the first 12 weeks of PTLD diagnosis.11-14 To our knowledge there are no clear data that suggest a higher rate of relapse with reinitiation of immune suppression after 12 weeks of remission. However, in patients who remained in remission for a few years and underwent retransplant along with reintroduction of immunosuppression, the risk of PTLD recurrence/relapse was minimal.15
The rationale for immunosuppressive therapy discontinuation before axi-cel infusion is to allow CAR T-cell expansion and persistence and avoidance of suppressing antitumor cytotoxic activity, all of which are crucial for efficacy. The lymphodepleting chemotherapy likely temporarily compensates for immunosuppressive therapy discontinuation during the first 6 to 8 weeks by decreasing the number of alloreactive lymphocytes.12,16 Additionally, targeting CD19 with CAR T-cell therapy can temporarily lead to B-cell aplasia and hypogammaglobulinemia, which, based on mouse studies, can lead to a significant reduction in kidney allograft rejection.17-19 The recovery of plasma and B cells thereafter, especially in the absence of a calcineurin inhibitor, puts patients at risk of developing antibody-mediated rejection. CAR T-cell proliferation peaks within the first 3 weeks of infusion, and having a higher peak in the first 28 days after axi-cel infusion was shown to correlate with objective response. In this regard, recent studies have shown early exposure to high dose and prolonged use of corticosteroids and calcineurin inhibitors may compromise CAR T-cell efficacy.20,21 Maintenance of response at the 3-month mark predicted long-term durability of response in about 80% of DLBCL patients.18 Furthermore, based on recent real world data of patients who received commercial axi-cel, the expected rate of complete remission was 64%, with 78% of patients who achieved a CR at day 30 maintaining that response at day 90.22 Although 2 of our 3 patients achieved a complete response at day 30, only 1 patient maintained response at month 3 and off immunosuppression.
Our series demonstrates the safety and feasibility of axi-cel in kidney transplant recipients with DLBCL. Based on our experience, immunosuppressive therapy can be safely stopped 2 to 4 weeks before leukapheresis to allow recovery of T-cell function and may be reinitiated 4 to 12 weeks after axi-cel in patients with ongoing remission, with close monitoring of kidney function (serial serum creatinine and dd-cfDNA levels).9 Nonetheless, the impact of restarting immunosuppression during this proposed time frame on decreasing the risk of graft rejection without increasing the risk of disease relapse has yet to be evaluated. Further studies are needed in this patient population to determine the optimal patient selection and timing for discontinuing and restarting immunosuppressive therapy.
For original data, please e-mail the corresponding author at sahmed3@mdanderson.org.
Acknowledgments
The University of Texas MD Anderson Cancer Center is supported, in part, by the National Institutes of Health, National Cancer Institute through Cancer Center Support Grant P30CA016672. Trial NCT03761056 was supported by Kite Pharma, a Gilead Company. The salary of P.S. is supported by the Lymphoma Research Foundation Career Development Award.
Authorship
Contribution: O.M. and R.N. wrote the manuscript; and S.P.I., A.E., S.S.N., R.E.S., S.A.A., M.H., N.S., K.D., P.S., S.M., and S.A. contributed patients and reviewed the manuscript.
Conflict-of-interest disclosure: S.I. received research grants and consults for Seattle Genetics, Rhizen, Daiichi Sankyo, and Trillium and receives research grants from Merck, Affimed, and Spectrum. S.S.N. served as an advisory board member or consultant for Kite, a Gilead Company, Merck, Bristol-Myers Squibb, Novartis, Celgene, Pfizer, Allogene Therapeutics, Cell Medica/Kuur, Incyte, Precision Biosciences, Legend Biotech, Adicet Bio, Calibr, and Unum Therapeutics; received research support from Kite, a Gilead Company, Bristol-Myers Squibb, Merck, Poseida, Cellectis, Celgene, Karus Therapeutics, Unum Therapeutics, Allogene Therapeutics, Precision Biosciences, and Acerta; received royalties from Takeda Pharmaceuticals; and has intellectual property related to cell therapy. The remaining authors declare no competing financial interests.
Correspondence: Sairah Ahmed, Department of Lymphoma/Myeloma, Division of Cancer Medicine, The University of Texas MD Anderson Cancer Center, 1400 Holcombe Blvd, Houston, TX 77030-4008; e-mail: sahmed3@mdanderson.org.
REFERENCES
Author notes
O.M. and R.N. contributed equally to this study.
S.M. and S.A. contributed equally to this study.