Angioimmunoblastic T-cell lymphoma (AITL), the most frequent subtype of peripheral T-cell lymphoma (PTCL), is a neoplasm with characteristics of mature T follicular helper (TFH) cells. We and others have identified frequent (~75%) inactivating mutations in the TET2 (Ten-Eleven Translocation-2) gene in AITL. TET2 belongs to a 3 member family of TET dioxygenases that catalyze DNA demethylation by oxidation of 5-methyl-cytosine (5-mC) to 5-hydroxymethyl-cytosine (5-hmC) and further oxidative cytosine products. Thus, loss of function (LOF) of TET2 will cause aberrant genome hypermethylation and reduction in 5-hmC. Studies of the variant allele fraction (VAF) of TET2 mutants suggest that this mutation is a founding abnormality in AITL. However, how TET2 loss promotes the development of AITL is still unclear.
To study LOF of TET2 in CD4 T-cell lymphomagenesis without the noise generated by other mutations in an established lymphoma, we generated a human TET2 knock-out (KO) CD4 T-cell model using CRISPR/Cas9 technology, which allows us to perform functional genomic studies by directly editing genes at their genomic loci. Whole transcriptome sequencing and single-cell transcriptome sequencing were used to study the cell evolution after KO.
We generated multiple TET2 KO primary CD4 T-cell models using two different CRISPR/Cas9 methods. The first approach used the plasmid PX458-a, which expresses green fluorescent protein (GFP) fused Cas9 and guide RNA-a targeting TET2 exon 6, to electroporate CD4 T-cell from healthy donor F25. The second approach used homologous DNA repair (HDR) mediated knock-in (KI) of tandem GFP gene and a SV40 transcription stop signal to terminate TET2 expression at exon 3. Cas9/sgRNA-e RNP complex, along with a long DNA template (about 1.6 kb), was electroporated into CD4 T-cells from two healthy donors, F25 and M40. GFP-positive cells were sorted by FACS after electroporation and were considered to be edited cells. Edited CD4 T-cells were cultured in vitro with 50 U/ml IL-2, and stimulated regularly (every 7~10 days) with 1:1 ratio of anti-CD3/CD28 T activator beads.
TET2 KO in these cells was confirmed by qRT-PCR, Sanger sequencing and Western blotting. Compared with wild-type (WT) CD4 T-cells under the same culture conditions, a lower level of 5-hmC in TET2 KO cells was observed, indicating successful editing of TET2.
Compared to WT cells, KO cells had a higher growth rate, due to a lower apoptosis rate and a higher proliferation rate, by Annexin V staining, EdU staining, and MTS experiments. The growth of KO cells or WT cells was still dependent on IL-2 and T activator beads stimulation. All batches of KO cells, generated by different guide RNAs or from different donors, showed a much longer life span than WT cells, which usually lived for 3~4 months, but KO cells can keep proliferating longer than one year.
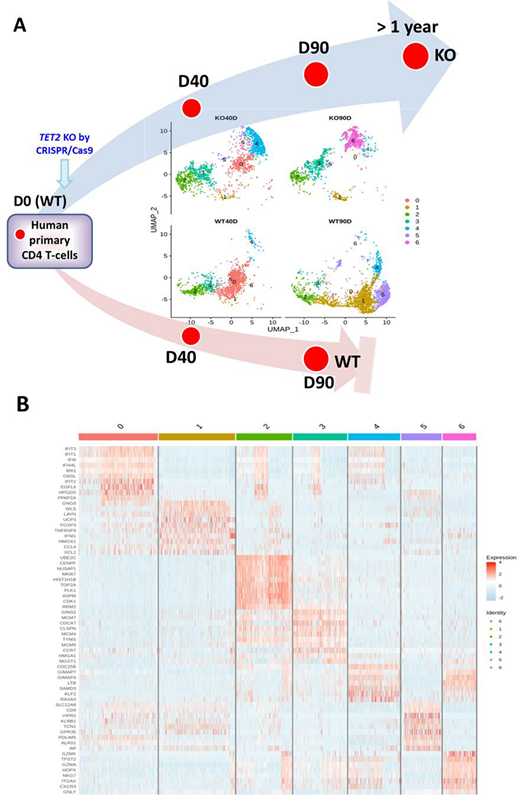
We also performed TCR analysis on these cell samples. Both WT and KO cells demonstrated oligoclonality when examined at Day 40 (40D, early stage) and TET2 KO cells showed a dominant clone by Day 90 (90D, late stage). We performed single-cell transcriptome analysis on M40 KO vs. WT cells, at 40D and 90D. KO90D cells had a low TCR diversity with the dominant population representing ~88% of cells (TRAV9-2,TRBV5-1). From single-cell transcriptome analysis, cell clustering profiles were very distinctive in these 4 cell populations analyzed (Figure 1A) and these clusters had unique gene expression profiles (Figure 1B). Cluster 6 was prominent in KO90D but almost absent in WT90D, whereas the reverse was true for clusters 1 and 5. From pathway analysis, KO90D cells showed a higher expression of signatures associated with proliferation, cell cycle and chemokine signaling and lower histidine and tryptophan metabolism signatures. Sanger sequencing showed a 79 bp indel in addition to the GFP KI allele in KO90D cells, demonstrated the homozygous deletion of TET2 on these cells. Similar results were observed in F25 TET2 KO cells by plasmid PX458-a.
This indicated the selection of homozygously deleted TET2 cells in long-term culture. However, clonal evolution is highly dynamic and a minor clone in KO40D cells may become the dominant clone in KO90D cells. Comparison of the 5-mC and 5-hmC profiles between KO and WT cells are being conducted to elucidate epigenetic alterations that are associated with the functional alterations and predisposition to AITL lymphomagenesis.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal