In this issue of Blood, Enns et al demonstrate that the mechanism of action of TMPRSS6, a key regulator of iron homeostasis, may differ from early predictions or assumptions.1 This finding has implications not only for our understanding of basic mechanisms of iron biology but also for the development of new pharmacologic agents that exploit or target TMPRSS6 to treat anemias and iron-related diseases.
TMPRSS6 has an impact on iron levels in the body by inhibiting hepcidin expression, which increases iron availability. Whether or not TMPRSS6 inhibits hepcidin expression by cleaving HJV and other proteins, or just by binding to these proteins, now needs to be reconsidered.
TMPRSS6 has an impact on iron levels in the body by inhibiting hepcidin expression, which increases iron availability. Whether or not TMPRSS6 inhibits hepcidin expression by cleaving HJV and other proteins, or just by binding to these proteins, now needs to be reconsidered.
TMPRSS6, also known as matriptase-2 (MT2), is a membrane protein abundantly expressed in the liver. It inhibits expression of hepcidin, a hormone made largely by the liver.2 Hepcidin inhibits dietary iron absorption and release of iron from cellular sites of iron storage. Hepcidin is abundantly expressed in conditions of iron excess and inflammation and minimally expressed in conditions of iron deficiency. By inhibiting an inhibitor, TMPRSS6 stimulates iron absorption and iron release from stores, thereby increasing the availability of iron throughout the body. This potent effect of TMPRSS6 on iron availability underscores the pivotal role of this protein in iron biology.
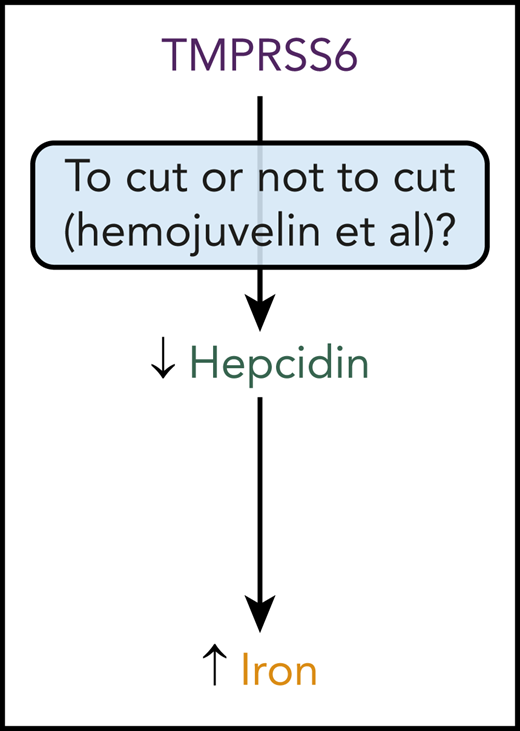
At the molecular level, it was originally thought that TMPRSS6 impacted hepcidin expression by binding to and cleaving key factors essential for hepcidin expression by the liver (see figure). These factors include hemojuvelin (HJV), a bone morphogenetic protein (BMP) coreceptor. HJV is essential for expression of hepcidin by the liver. Mutations in HJV cause a juvenile form of hereditary hemochromatosis, a common disease of iron excess. With HJV deficiency, the liver is unable to express sufficient amounts of hepcidin, and dietary iron absorption continues unabated. By cleaving and inactivating HJV, TMPRSS6 downregulates hepcidin expression by the liver. Mutations in TMPRSS6 cause a disease known as iron-refractory iron deficiency anemia or IRIDA. In this disease, TMPRSS6 deficiency leads to excessive hepcidin expression and suppression of dietary iron absorption. The anemia observed in these patients is refractory to administration of oral iron because of limited dietary iron absorption.
In their article, Enns et al demonstrate, by using cell culture- and mouse-based studies, that mutant forms of TMPRSS6 lacking the ability to cleave proteins can still suppress hepcidin expression (see figure). They provide further evidence that the extracellular domain of TMPRSS6 is essential for suppression of hepcidin expression by these mutant forms of TMPRSS6 and that this domain binds to HJV and other factors essential for hepcidin expression. Overall, this work suggests that the ability of TMPRSS6 to bind other proteins, but not cleave other proteins, is a key determinant of its function. What remains to be determined is how TMPRSS6 binding to specific proteins impacts hepcidin expression. Presumably, TMPRSS6 binding impedes HJV and other proteins from stimulating hepcidin expression, but this has yet to be shown in detail at a mechanistic level.
The findings of Enns et al have clinical implications for treating anemia and iron-related diseases. Suppression of Tmprss6 expression attenuates the severity of disease in mouse models of β-thalassemia and hereditary hemochromatosis, whereas chemical inhibition of TMPRSS6 activity stimulates hepcidin expression in cell lines.3-9 Tmprss6 suppression in the mouse models of disease increases hepcidin levels and limits dietary iron absorption and release of iron from cellular stores; limiting iron levels in β-thalassemia is believed to improve anemia by limiting iron-mediated toxicity in red cell precursors. Pharmacologic suppression of TMPRSS6 expression is currently being explored as a potential treatment for β-thalassemia in clinical trials.10 The findings by Enns et al suggest that targeting TMPRSS6 function should focus not on the proteolytic activity of TMPRSS6 but rather on its ability to bind proteins such as HJV. This could lead to the generation of a new type of pharmacologic agent that scientists could use to better understand the mechanistic underpinnings of iron biology and that physicians could use to treat patients with iron-loading anemias, hereditary hemochromatosis, and other iron-related diseases.
Conflict-of-interest disclosure: The author declares no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal