Visual Abstract
Professional illustration by Patrick Lane, ScEYEnce Studios
Abstract
Despite increasing use of targeted therapies to treat cancer, anemia remains a common complication of cancer therapy. Physician concerns about the safety of intravenous (IV) iron products and erythropoiesis-stimulating agents (ESAs) have resulted in many patients with cancer receiving no or suboptimal anemia therapy. In this article, we present 4 patient cases that illustrate both common and complex clinical scenarios. We first present a review of erythropoiesis and then describe our approach to cancer-associated anemia by identifying the contributing causes before selecting specific treatments. We summarize clinical trial data affirming the safety and efficacy of currently available IV iron products used to treat cancer-associated anemia and illustrate how we use commonly available laboratory tests to assess iron status during routine patient management. We compare adverse event rates associated with IV iron vs red cell transfusion and discuss using first-line IV iron monotherapy to treat anemic patients with cancer, which decreases the need for ESAs. A possible mechanism behind ESA-induced tumor progression is discussed. Finally, we review the potential of novel therapies such as ascorbic acid, prolyl hydroxylase inhibitors, activin traps, hepcidin, and bone morphogenetic protein antagonists in treating cancer-associated anemia.
Introduction
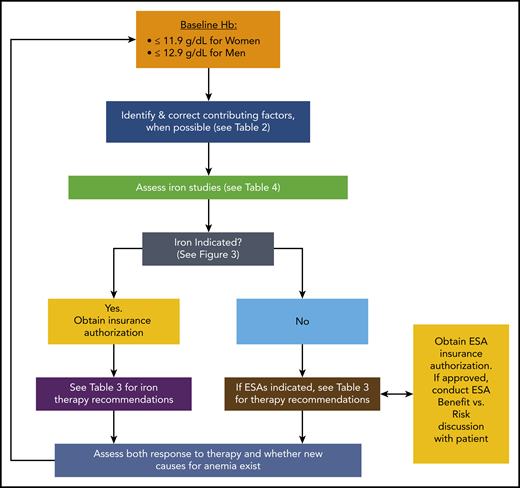
During the past 5 years, more than 50 new agents for use in the anticancer armamentarium have been added to the traditional myelosuppressive chemotherapy drugs still being used.1 Despite these advances, off-target effects of newer, antineoplastic therapies (ANTs) such as tyrosine kinase inhibitors, monoclonal antibody conjugates, and immunomodulating agents persist, and treatment-related anemia remains a common clinical problem. The prevalence of anemia can exceed 90% for those receiving certain treatments.2-5 It often has a negative impact on quality of life (QOL), and it can limit a person’s ability to perform activities of daily living,6,7 including self-care functions and the ability to work. Fatigue, now recognized as the most distressing symptom reported by patients with cancer, is often intimately tied to anemia. Moreover, anemia can be a dose-limiting toxicity that prevents patients from realizing the full benefit from ANTs. Variables such as sex, age, cancer type, stage, and type of ANT, as well as concomitant medications and comorbidities may all contribute to cancer-associated anemia, which makes broad treatment recommendations difficult, if not impossible. Therefore, identifying specific causes of cancer-associated anemia is necessary for developing effective treatment strategies. Herein we describe our approach to the diagnosis and treatment of cancer-associated anemia and present both common and uniquely challenging cases. A summary of our approach is depicted in Figure 1.
An overview of our approach to the diagnosis and management of cancer-associated anemia. For more detail regarding specific steps, see the referenced tables and figures.
An overview of our approach to the diagnosis and management of cancer-associated anemia. For more detail regarding specific steps, see the referenced tables and figures.
How do we define cancer-associated anemia?
We define anemia as cancer-associated anemia when it results from malignancy or its treatment.8 We follow the World Health Organization definition for anemia based upon the patient’s sex.9 Clinical trials may report the incidence of cancer-associated anemia using the US Food and Drug Administration’s Common Terminology Criteria for Adverse Events (CTCAE),10 which offers an anemia grading system. However, the CTCAE fails to define the lower limit of a normal hemoglobin (Hb) concentration for either sex. By incorporating guidance from both the World Health Organization and CTCAE, we can construct a definition for cancer-associated anemia that reconciles shortcomings (Table 1). When examining clinical data, note that trials may report only grade 3 to 4 anemia.11,12
Definition and criteria for grading cancer-associated anemia
| Anemia grade . | CTCAE + WHO Criteria . | |
|---|---|---|
| Women . | Men . | |
| Grade 1 | Hb 11.9-10.0 g/dL | Hb 12.9-10.0 g/dL |
| Grade 2 | Hb 9.9-8.0 g/dL | Hb 9.9-8.0 g/dL |
| Grade 3 | Hb ≤7.9 g/dL | Hb <7.9 g/dL |
| Grade 4 | Life-threatening consequences requiring urgent intervention, such as RBC transfusion | Life-threatening consequences requiring urgent intervention, such as RBC transfusion |
| Anemia grade . | CTCAE + WHO Criteria . | |
|---|---|---|
| Women . | Men . | |
| Grade 1 | Hb 11.9-10.0 g/dL | Hb 12.9-10.0 g/dL |
| Grade 2 | Hb 9.9-8.0 g/dL | Hb 9.9-8.0 g/dL |
| Grade 3 | Hb ≤7.9 g/dL | Hb <7.9 g/dL |
| Grade 4 | Life-threatening consequences requiring urgent intervention, such as RBC transfusion | Life-threatening consequences requiring urgent intervention, such as RBC transfusion |
By using World Health Organization (WHO) criteria and the CTCAE (v5.0) grading system for anemia, a definition for cancer-associated anemia was established: grade 1 anemia was defined as Hb ≤11.9 g/dL for women with cancer and Hb ≤12.9 g/dL for men with cancer. Severity of anemia increases with increasing grade up to grade 5 (death).
Erythropoiesis
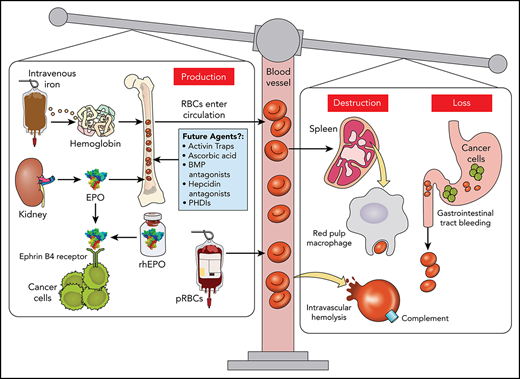
The coordinated daily synthesis of billions of red blood cells (RBCs) requires complex interactions and feedback mechanisms between the gut, liver, spleen, kidney, and bone marrow.13 The initial work-up of cancer-associated anemia may require evaluating many of these pathways (Figure 2).
Pathophysiology of cancer-associated anemia. (A) EPO transcription is dependent upon HIF-2⍺, which under normoxic (physiologic oxygenation) conditions is controlled by proteasomal degradation of HIF-2⍺ through a ubiquitination (Ub) process mediated by the von Hippel-Lindau (vHL) protein. With hypoxia, prolyl hydroxylases (PHD) are unable to hydroxylate proline residues on HIF-2α required for vHL binding. This permits HIF-2⍺ to accumulate, dimerize with HIF-1β and bind the EPO promoter region of DNA in renal EPO-producing cells. Secreted EPO migrates to the bone marrow, and upon binding to the EPO receptor located on early erythroid cells (colony forming units-erythroid and proerythroblasts), induces dimerization and phosphorylation of its Janus kinase receptor, leading to erythroblast proliferation.6 Erythrocyte production is closely regulated by the concentration of transferrin-bound iron and may also be diminished by certain anti-neoplastic agents, radiotherapy, or cancer itself.14,15 Upon division, basophilic and polychromatophilic erythroblasts are capable of secreting erythroferrone (ERFE).16 (B) Regulation of iron metabolism involves several rate-limiting steps affecting RBC development. The key regulator of iron metabolism is hepcidin, a small peptide that inhibits iron flux, in both the gastrointestinal tract and splenic macrophages. EPO-dependent induction of ERFE produced by erythroid precursors during stress erythropoiesis functions to suppress hepcidin transcription via bone morphogenetic proteins BMP-5, -6, and -7 to allow for rapid recovery from blood loss.16-18 Hepcidin production is also regulated by iron, with iron excess increasing hepcidin expression (resulting in hypoferremia), and iron deficiency decreasing hepcidin expression.19,20 Hepcidin production is further regulated by inflammation, with cytokines such as interleukin-6 increasing hepcidin synthesis via STAT3 upregulation.21,22 Iron-dependent induction of hepcidin requires hemojuvelin (HJV), a BMP co-receptor, also found on the hepatocyte.19 When serum iron concentrations rise and with the help of HJV, BMP-6 and to some extent BMP-2, bind to their cognate receptor to activate HAMP gene (encodes hepcidin) transcription via the SMAD pathway.19 HFE (high iron) and TFR2 also play a role in BMP signaling.23-25 Hepcidin transcription is reduced when the transmembrane serine protease TMPRSS6 (also known as matriptase-2) cleaves HJV to deactivate the BMP receptor (BMPR). (C) Dietary iron, once reduced (Fe2+), is absorbed via the divalent metal transporter 1 (DMT1) on the apical surface of the intestinal lumen.22 However, once inside this cell, hepcidin, located on the opposite basolateral surface, regulates iron egress into the bloodstream. Hephaestin (not shown) oxidizes Fe2+ to Fe3+ which is required by transferrin for iron docking.22 Inhibition of iron flux occurs when hepcidin binds to ferroportin of gastrointestinal cells and macrophages, leading to ferroportin internalization and degradation.26 (D) Increased levels of hepcidin block mobilization of iron from storage sites, including macrophages in the red pulp of the spleen. (E) Exogenous rhEPO or drug-induced (eg, roxadustat, a prolyl hydroxylase inhibitor [PHDI]) endogenously-produced EPO may bind to the ephrin-B4 receptor on tumor tissue to promote tumor growth. Professional illustration by Patrick Lane, ScEYEnce Studios.
Pathophysiology of cancer-associated anemia. (A) EPO transcription is dependent upon HIF-2⍺, which under normoxic (physiologic oxygenation) conditions is controlled by proteasomal degradation of HIF-2⍺ through a ubiquitination (Ub) process mediated by the von Hippel-Lindau (vHL) protein. With hypoxia, prolyl hydroxylases (PHD) are unable to hydroxylate proline residues on HIF-2α required for vHL binding. This permits HIF-2⍺ to accumulate, dimerize with HIF-1β and bind the EPO promoter region of DNA in renal EPO-producing cells. Secreted EPO migrates to the bone marrow, and upon binding to the EPO receptor located on early erythroid cells (colony forming units-erythroid and proerythroblasts), induces dimerization and phosphorylation of its Janus kinase receptor, leading to erythroblast proliferation.6 Erythrocyte production is closely regulated by the concentration of transferrin-bound iron and may also be diminished by certain anti-neoplastic agents, radiotherapy, or cancer itself.14,15 Upon division, basophilic and polychromatophilic erythroblasts are capable of secreting erythroferrone (ERFE).16 (B) Regulation of iron metabolism involves several rate-limiting steps affecting RBC development. The key regulator of iron metabolism is hepcidin, a small peptide that inhibits iron flux, in both the gastrointestinal tract and splenic macrophages. EPO-dependent induction of ERFE produced by erythroid precursors during stress erythropoiesis functions to suppress hepcidin transcription via bone morphogenetic proteins BMP-5, -6, and -7 to allow for rapid recovery from blood loss.16-18 Hepcidin production is also regulated by iron, with iron excess increasing hepcidin expression (resulting in hypoferremia), and iron deficiency decreasing hepcidin expression.19,20 Hepcidin production is further regulated by inflammation, with cytokines such as interleukin-6 increasing hepcidin synthesis via STAT3 upregulation.21,22 Iron-dependent induction of hepcidin requires hemojuvelin (HJV), a BMP co-receptor, also found on the hepatocyte.19 When serum iron concentrations rise and with the help of HJV, BMP-6 and to some extent BMP-2, bind to their cognate receptor to activate HAMP gene (encodes hepcidin) transcription via the SMAD pathway.19 HFE (high iron) and TFR2 also play a role in BMP signaling.23-25 Hepcidin transcription is reduced when the transmembrane serine protease TMPRSS6 (also known as matriptase-2) cleaves HJV to deactivate the BMP receptor (BMPR). (C) Dietary iron, once reduced (Fe2+), is absorbed via the divalent metal transporter 1 (DMT1) on the apical surface of the intestinal lumen.22 However, once inside this cell, hepcidin, located on the opposite basolateral surface, regulates iron egress into the bloodstream. Hephaestin (not shown) oxidizes Fe2+ to Fe3+ which is required by transferrin for iron docking.22 Inhibition of iron flux occurs when hepcidin binds to ferroportin of gastrointestinal cells and macrophages, leading to ferroportin internalization and degradation.26 (D) Increased levels of hepcidin block mobilization of iron from storage sites, including macrophages in the red pulp of the spleen. (E) Exogenous rhEPO or drug-induced (eg, roxadustat, a prolyl hydroxylase inhibitor [PHDI]) endogenously-produced EPO may bind to the ephrin-B4 receptor on tumor tissue to promote tumor growth. Professional illustration by Patrick Lane, ScEYEnce Studios.
Diagnosis of cancer-associated anemia
Cancer-associated anemia is caused by 1 or more of 3 primary mechanisms: (1) ineffective erythropoiesis,4,27 (2) hemolysis, or (3) blood loss.28 Our approach subclassifies potential causes of cancer-associated anemia by first listing 3 broad categories: production, destruction, and loss (bleeding). Further subgrouping creates 3 additional categories: drug-induced, infectious-related, or diseases or disorders/other-based causes, re-named as Drugs, Bugs, and Diseases in Table 2.
Potential etiologies for cancer-associated anemia
| Causes of decreased Hb . | Production (decreased) . | Destruction (increased) . | Loss (overt, occult, or iatrogenic bleeding) . |
|---|---|---|---|
| Drugs/therapy | Myelosuppressive chemotherapy | Antibiotics (eg, β-lactams, dapsone) | Anticoagulation (eg, DOACs, LMWHs, warfarin) |
| Radiation therapy | Chemotherapy (eg, gemcitabine) | Antiplatelet agents (eg, clopidogrel, prasugrel, ticagrelor) | |
| Tyrosine kinase inhibitors (delayed maturation) | Immunotherapy (eg, nivolumab, pembrolizumab, ipilimumab) | Nonsteroidal anti-inflammatory drugs | |
| Immunotherapy (inflammation) | Intravenous immunoglobulin G | Over-the-counter supplements (eg, turmeric) | |
| Bugs (bacteria, virus, or fungi) | Cytomegalovirus | HIV | Helicobacter pylori |
| Parvovirus B19 (in SCT or immunocompromised patients) | |||
| Diseases, disorders, or other | Chronic kidney disease (decreased erythropoietin) | Microangiopathic hemolytic anemia (may be drug- or disease-related) | Gastrointestinal tumors |
| Clonal hematopoiesis of indeterminate potential | Frequent phlebotomy | ||
| Iron deficiency (AIDA or FIDA) | Autoimmune hemolytic anemia (eg, caused by autoimmune disorders or drug-induced) | Arteriovenous malformations | |
| Leukemia (acute or chronic) | Hemostatic disorders (inherited or acquired) | ||
| De novoor therapy-related myelodysplastic syndromes | Menses | ||
| Myeloproliferative neoplasms | Hemophagocytic lymphohistiocytosis | Surgery | |
| Solid tumors | Disseminated intravascular coagulation | ||
| Iron overload | |||
| B9 (folate) or B12 (cobalamin) deficiency |
| Causes of decreased Hb . | Production (decreased) . | Destruction (increased) . | Loss (overt, occult, or iatrogenic bleeding) . |
|---|---|---|---|
| Drugs/therapy | Myelosuppressive chemotherapy | Antibiotics (eg, β-lactams, dapsone) | Anticoagulation (eg, DOACs, LMWHs, warfarin) |
| Radiation therapy | Chemotherapy (eg, gemcitabine) | Antiplatelet agents (eg, clopidogrel, prasugrel, ticagrelor) | |
| Tyrosine kinase inhibitors (delayed maturation) | Immunotherapy (eg, nivolumab, pembrolizumab, ipilimumab) | Nonsteroidal anti-inflammatory drugs | |
| Immunotherapy (inflammation) | Intravenous immunoglobulin G | Over-the-counter supplements (eg, turmeric) | |
| Bugs (bacteria, virus, or fungi) | Cytomegalovirus | HIV | Helicobacter pylori |
| Parvovirus B19 (in SCT or immunocompromised patients) | |||
| Diseases, disorders, or other | Chronic kidney disease (decreased erythropoietin) | Microangiopathic hemolytic anemia (may be drug- or disease-related) | Gastrointestinal tumors |
| Clonal hematopoiesis of indeterminate potential | Frequent phlebotomy | ||
| Iron deficiency (AIDA or FIDA) | Autoimmune hemolytic anemia (eg, caused by autoimmune disorders or drug-induced) | Arteriovenous malformations | |
| Leukemia (acute or chronic) | Hemostatic disorders (inherited or acquired) | ||
| De novoor therapy-related myelodysplastic syndromes | Menses | ||
| Myeloproliferative neoplasms | Hemophagocytic lymphohistiocytosis | Surgery | |
| Solid tumors | Disseminated intravascular coagulation | ||
| Iron overload | |||
| B9 (folate) or B12 (cobalamin) deficiency |
This table represents the authors’ approach to defining etiologies of cancer-associated anemia. The main categories are production defects, destruction, and blood loss. Examples of each category are provided in the columns. This table does not represent a comprehensive list of all possible causes of cancer-associated anemia.
DOAC, direct oral anticoagulant; LMWH, low molecular weight heparin; SCT, stem cell transplantation.
Goals of therapy for cancer-associated anemia
The short-term goal is correcting the quantitative deficits of Hb and erythrocytes to meet the oxygenation requirements of all tissues. If successful, meeting these goals also translates into increased QOL through improvement in cognition, fatigue, and exercise tolerance.7 Depending upon cancer stage and prognosis, goals may shift from correction of cancer-associated anemia to maintaining QOL by preventing worsening anemia and dependency on RBC transfusion.
Currently available treatments focus on increasing RBC production to counterbalance treatment-induced effects on production (Table 3). Particular attention is paid to RBC destruction and blood loss because they can often be addressed by targeting the underlying disease or screening for concomitant medications that are exacerbating blood loss (Table 2). Importantly, fewer than half of all patients with anemia receive treatment with drugs, potentially sparked by a precipitous decline in the use of erythropoiesis-stimulating agents (ESAs) that began in 2006.29,30 Unfortunately, no single therapy can be offered to all patients with cancer-associated anemia. With this background, we present 4 cases illustrating how we manage cancer-associated anemia.
Treatment options for managing anemia of cancer or chemotherapy
| . | Dosage . | Cancer indication . | Comments . |
|---|---|---|---|
| IV iron products | |||
| Ferric gluconate | 125 mg over 60 minutes | No | Associated with serious infusion reactions |
| Repeat doses over 2 to 3 weeks to achieve 1000-mg total dose | |||
| Ferric carboxymaltose | 750 mg over 7.5 minutes as slow IV push or over ≥15 minutes as infusion | No | Broad indication for iron deficiency |
| May consider a repeat dose in ≥7 days | Transient hypophosphatemia is a common adverse reaction | ||
| Ferumoxytol | 510 mg over ≥15 minutes | No | Broad indication for iron deficiency |
| Repeat dose in ≥3 days | Affects MRI interpretation | ||
| Black-box warning for hypersensitivity | |||
| Ferric derisomaltose (also known as iron isomaltoside) | For patients ≥50 kg give 1000 mg over ≥20 minutes. For patients weighing <50 kg give 20 mg/kg using actual body weight over ≥20 minutes | No | Broad indication for iron deficiency |
| Up to 1500 mg in divided doses (eg, 1000 mg + 500 mg). A dose of 500 mg can be given over ~2 minutes ≥7 days after initial dose | Approved in the United States in early 2020 | ||
| Iron sucrose | 200 mg over 5 minutes or 300 mg over 90 minutes | No | Most commonly used IV iron product in the United States |
| Repeat dosing over 3 to 5 weeks to achieve 900- to 1000-mg total dose target | |||
| LMW iron dextran | 1000 mg over 1 to 2 hours most common | No | Broad indication for iron deficiency |
| Dosing range, 100 to 2000 mg | Black-box warning for hypersensitivity | ||
| Doses exceeding 100 mg are not FDA-approved but have been the generally accepted practice for more than 3 decades | Test dose (25 mg/0.5 mL) required before the first infusion | ||
| Doses up to 100 mg may be infused undiluted, rate not to exceed 50 mg/minute | |||
| Total dose infusion (up to 2000 mg per dose) possible | |||
| Oral iron products | |||
| Oral iron salts may vary. | Ferrous sulfate 325 mg (65 mg elemental) orally once per day to 3 times per day, as tolerated. | No | May use up to 195 mg elemental iron daily; dose and frequency vary per patient tolerance. Preparations containing less elemental iron per dose may cause less gastrointestinal upset |
| ESAs | |||
| Epoetin alfa (and biosimilars) | Practice-based fixed dosing: 40 000 units SC once per week or 80 000 units SC once every 2 weeks or 120 000 units SC once every 3 weeks | Yes | ESAs indicated for chemotherapy-induced anemia, not anemia from cancer alone |
| FDA-approved dosing: 150 units/kg SC 3 times per week or 40 000 units SC once per week | Use the lowest dose for patients with chronic kidney disease–induced anemia and consider risk of tumor progression in this population | ||
| Darbepoetin alfa | Practice-based fixed dosing: 200 μg SC once every 2 weeks or 300 μg SC once every 3 weeks or 500 μg SC once every 3 weeks | Yes | ESAs indicated for chemotherapy-induced anemia, not anemia from cancer alone |
| FDA-approved dosing: 2.25 μg/kg SC once every week or 500 μg SC once every 3 weeks | Use the lowest dose for patients with chronic kidney disease–induced anemia and consider risk of tumor progression in this population | ||
| B vitamins | |||
| Folic acid (vitamin B9) | 1 mg orally once per day | No | May use over-the-counter preparations |
| Cyanocobalamin (vitamin B12) | 1000 μg SC (deep) or intramuscular injection on days 1,3,7,10, 14, 21, 30, and every 30 days thereafter or 2000 μg orally once per day | No | Schedule of parenteral cyanocobalamin is variable.31 Intramuscular/SC vitamin B12 can be self-administered at home |
| Androgens | |||
| Testosterone | Dosage variable and based upon formulation; available as oral, buccal, topical (gel, solution, or patch), intranasal, subcutaneous pellet, and intramuscular preparations | No | Used in myeloproliferative neoplasm and bone marrow transplant patients. Consider topical route in patients with thrombocytopenia. Response typically seen within 3 months |
| Danazol | 200 mg orally 3 times per day or 400 mg orally twice per day | No | Used in patients with myeloproliferative neoplasms; response typically seen within 3 months |
| . | Dosage . | Cancer indication . | Comments . |
|---|---|---|---|
| IV iron products | |||
| Ferric gluconate | 125 mg over 60 minutes | No | Associated with serious infusion reactions |
| Repeat doses over 2 to 3 weeks to achieve 1000-mg total dose | |||
| Ferric carboxymaltose | 750 mg over 7.5 minutes as slow IV push or over ≥15 minutes as infusion | No | Broad indication for iron deficiency |
| May consider a repeat dose in ≥7 days | Transient hypophosphatemia is a common adverse reaction | ||
| Ferumoxytol | 510 mg over ≥15 minutes | No | Broad indication for iron deficiency |
| Repeat dose in ≥3 days | Affects MRI interpretation | ||
| Black-box warning for hypersensitivity | |||
| Ferric derisomaltose (also known as iron isomaltoside) | For patients ≥50 kg give 1000 mg over ≥20 minutes. For patients weighing <50 kg give 20 mg/kg using actual body weight over ≥20 minutes | No | Broad indication for iron deficiency |
| Up to 1500 mg in divided doses (eg, 1000 mg + 500 mg). A dose of 500 mg can be given over ~2 minutes ≥7 days after initial dose | Approved in the United States in early 2020 | ||
| Iron sucrose | 200 mg over 5 minutes or 300 mg over 90 minutes | No | Most commonly used IV iron product in the United States |
| Repeat dosing over 3 to 5 weeks to achieve 900- to 1000-mg total dose target | |||
| LMW iron dextran | 1000 mg over 1 to 2 hours most common | No | Broad indication for iron deficiency |
| Dosing range, 100 to 2000 mg | Black-box warning for hypersensitivity | ||
| Doses exceeding 100 mg are not FDA-approved but have been the generally accepted practice for more than 3 decades | Test dose (25 mg/0.5 mL) required before the first infusion | ||
| Doses up to 100 mg may be infused undiluted, rate not to exceed 50 mg/minute | |||
| Total dose infusion (up to 2000 mg per dose) possible | |||
| Oral iron products | |||
| Oral iron salts may vary. | Ferrous sulfate 325 mg (65 mg elemental) orally once per day to 3 times per day, as tolerated. | No | May use up to 195 mg elemental iron daily; dose and frequency vary per patient tolerance. Preparations containing less elemental iron per dose may cause less gastrointestinal upset |
| ESAs | |||
| Epoetin alfa (and biosimilars) | Practice-based fixed dosing: 40 000 units SC once per week or 80 000 units SC once every 2 weeks or 120 000 units SC once every 3 weeks | Yes | ESAs indicated for chemotherapy-induced anemia, not anemia from cancer alone |
| FDA-approved dosing: 150 units/kg SC 3 times per week or 40 000 units SC once per week | Use the lowest dose for patients with chronic kidney disease–induced anemia and consider risk of tumor progression in this population | ||
| Darbepoetin alfa | Practice-based fixed dosing: 200 μg SC once every 2 weeks or 300 μg SC once every 3 weeks or 500 μg SC once every 3 weeks | Yes | ESAs indicated for chemotherapy-induced anemia, not anemia from cancer alone |
| FDA-approved dosing: 2.25 μg/kg SC once every week or 500 μg SC once every 3 weeks | Use the lowest dose for patients with chronic kidney disease–induced anemia and consider risk of tumor progression in this population | ||
| B vitamins | |||
| Folic acid (vitamin B9) | 1 mg orally once per day | No | May use over-the-counter preparations |
| Cyanocobalamin (vitamin B12) | 1000 μg SC (deep) or intramuscular injection on days 1,3,7,10, 14, 21, 30, and every 30 days thereafter or 2000 μg orally once per day | No | Schedule of parenteral cyanocobalamin is variable.31 Intramuscular/SC vitamin B12 can be self-administered at home |
| Androgens | |||
| Testosterone | Dosage variable and based upon formulation; available as oral, buccal, topical (gel, solution, or patch), intranasal, subcutaneous pellet, and intramuscular preparations | No | Used in myeloproliferative neoplasm and bone marrow transplant patients. Consider topical route in patients with thrombocytopenia. Response typically seen within 3 months |
| Danazol | 200 mg orally 3 times per day or 400 mg orally twice per day | No | Used in patients with myeloproliferative neoplasms; response typically seen within 3 months |
FDA, US Food and Drug Administration; MRI, magnetic resonance imaging; SC, subcutaneously.
Case 1
Patient 1, a 58-year-old man presenting with intermittent hematochezia, is diagnosed with low-risk stage III adenocarcinoma of the colon. He receives adjuvant therapy with folinic acid, fluorouracil, and oxaliplatin (FOLFOX) every 14 days for 6 months. His tumor is considered curable. Baseline laboratory results show that Hb was 11.6 g/dL, and results from iron studies showed ferritin 9 ng/mL, serum iron 41 μg/dL, total iron binding capacity 438 μg/dL, and transferrin saturation (TSAT) 9%. Normal iron reference intervals are shown in Table 4. How should this patient's anemia be treated?
Laboratory tests used to evaluate iron status
| Test . | Sex . | Reference interval . | Comments . |
|---|---|---|---|
| Serum ferritin | M | 30-500 ng/mL | A low value indicates absolute iron deficiency. In a patient with liver disease or inflammation (eg, cancer), a normal or increased value does not exclude iron deficiency. |
| F | 12-240 ng/mL | ||
| TSAT | 20%-50% | Low values indicate iron-restricted erythropoiesis and iron deficiency. A low ferritin and low TSAT indicate absolute iron deficiency. A normal or elevated ferritin and low TSAT indicate iron sequestration. | |
| Serum iron | M | 50-170 μg/dL | |
| F | 30-160 μg/dL | ||
| TIBC | 240-450 μg/dL | ||
| ZPP | <70 μmol/mol heme | Elevated levels are also seen in lead poisoning, sideroblastic anemia, and other disorders. | |
| sTfR | M | 2.2-5.0 mg/L | sTfR is unaffected by inflammation, but the test has poor predictive value in the cancer population. |
| F | 1.9-4.4 mg/L | ||
| Hepcidin | Optimal threshold for response yet to be defined; it is likely <64.3 ng/mL (baseline) based upon currently available data | Baseline hepcidin level seems to strongly correlate with response to IV iron in FIDA; however, this test is not routinely available. | |
| MCV | M | 83-98 fL | Not sensitive or specific for iron deficiency. Affected by drugs, liver disease, and other factors. |
| F | 85-98 fL* | ||
| Reticulocyte hemoglobin content | Not routinely available | May be helpful in determining when patients will benefit from IV iron. |
| Test . | Sex . | Reference interval . | Comments . |
|---|---|---|---|
| Serum ferritin | M | 30-500 ng/mL | A low value indicates absolute iron deficiency. In a patient with liver disease or inflammation (eg, cancer), a normal or increased value does not exclude iron deficiency. |
| F | 12-240 ng/mL | ||
| TSAT | 20%-50% | Low values indicate iron-restricted erythropoiesis and iron deficiency. A low ferritin and low TSAT indicate absolute iron deficiency. A normal or elevated ferritin and low TSAT indicate iron sequestration. | |
| Serum iron | M | 50-170 μg/dL | |
| F | 30-160 μg/dL | ||
| TIBC | 240-450 μg/dL | ||
| ZPP | <70 μmol/mol heme | Elevated levels are also seen in lead poisoning, sideroblastic anemia, and other disorders. | |
| sTfR | M | 2.2-5.0 mg/L | sTfR is unaffected by inflammation, but the test has poor predictive value in the cancer population. |
| F | 1.9-4.4 mg/L | ||
| Hepcidin | Optimal threshold for response yet to be defined; it is likely <64.3 ng/mL (baseline) based upon currently available data | Baseline hepcidin level seems to strongly correlate with response to IV iron in FIDA; however, this test is not routinely available. | |
| MCV | M | 83-98 fL | Not sensitive or specific for iron deficiency. Affected by drugs, liver disease, and other factors. |
| F | 85-98 fL* | ||
| Reticulocyte hemoglobin content | Not routinely available | May be helpful in determining when patients will benefit from IV iron. |
Reference intervals are taken from ARUP Laboratories; zinc protoporphyrin (ZPP) from Mayo Clinic Laboratories.
F, female; M, male; sTfR, soluble transferrin receptor; TIBC, total iron binding capacity.
MCV reference intervals are from Wakeman et al.32
Iron deficiency
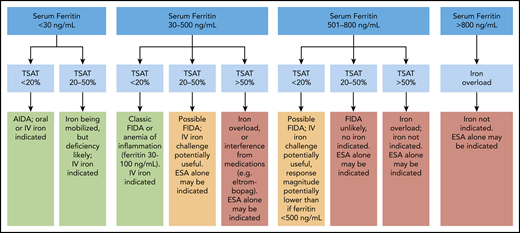
Three types of iron-deficiency anemia syndromes have been described: (1) absolute iron deficiency anemia (AIDA; usually associated with bleeding) in which total body iron stores are very low, (2) iron sequestration related to inflammation in which hepcidin-mediated iron block renders iron unavailable for erythropoiesis, and (3) iron-restricted erythropoiesis in which endogenous erythropoietin (EPO) or ESA therapy induces erythropoiesis that outpaces iron delivery from storage.33 The latter 2 categories can be considered examples of functional iron deficiency anemia (FIDA) (Figure 3).
Algorithm using serum ferritin and transferrin saturation to predict response to iron in cancer-associated anemia. Green boxes indicate benefits of IV iron therapy. Yellow boxes indicate that an iron trial may be beneficial. Red boxes indicate that iron should not be given. Patients with TSAT <20% and inflammation elevating the serum ferritin (up to 100 ng/mL) will likely respond in a manner similar to that of classic AIDA; patients with ferritin >100 ng/mL may exhibit slightly lower Hb responses. A CHr and reticulocyte count may help to determine whether to give iron in this instance. Professional illustration by Patrick Lane, ScEYEnce Studios.
Algorithm using serum ferritin and transferrin saturation to predict response to iron in cancer-associated anemia. Green boxes indicate benefits of IV iron therapy. Yellow boxes indicate that an iron trial may be beneficial. Red boxes indicate that iron should not be given. Patients with TSAT <20% and inflammation elevating the serum ferritin (up to 100 ng/mL) will likely respond in a manner similar to that of classic AIDA; patients with ferritin >100 ng/mL may exhibit slightly lower Hb responses. A CHr and reticulocyte count may help to determine whether to give iron in this instance. Professional illustration by Patrick Lane, ScEYEnce Studios.
In 2004, a publication by Auerbach et al34 first reported treatment of iron deficiency in patients with chemotherapy-induced, cancer-associated anemia. Intravenous (IV) iron was found to favorably augment the efficacy of ESAs in patients with solid tumors and non-myeloid malignancies.34 Subsequently, numerous clinical trials35-37 have confirmed the efficacy of adding iron to ESA therapy.
Iron studies
The most widely available and frequently used tests to assess iron status are serum ferritin and TSAT percentage ([serum iron/total iron binding capacity] × 100). Ferritin is a biomarker of total body iron stores, and when it is decreased, low ferritin is a reliable indicator of AIDA. However, ferritin is an acute-phase reactant, and in conditions that cause inflammation such as cancer, the ferritin result may not reliably quantify iron stores.38 TSAT is an indicator of iron availability for erythropoiesis. Low TSAT percentages (<20%) suggest functional iron deficiency (FID) when serum ferritin is >100 ng/mL.39 Combining the results of serum ferritin and TSAT tests is useful for defining a patient’s iron status and suggesting treatment options (Figure 3).39
Other laboratory tests for evaluating iron status include zinc protoporphyrin, soluble transferrin receptor, hepcidin, mean corpuscular volume (MCV), and reticulocyte hemoglobin content (CHr) (Table 4). However, compared with serum ferritin and TSAT tests, these other assays have limitations. For example, an elevated zinc protoporphyrin level is not specific for iron deficiency; soluble transferrin receptor has poor predictive value,40 and hepcidin assays are not widely available. Decreased MCV levels occur late in iron deficiency, and this parameter can be elevated in numerous other conditions, thus masking true iron deficiency. CHr is a promising test for evaluating iron status, but this analyte is not routinely reported with complete blood counts.
Iron therapy options
The route chosen for iron repletion (oral vs IV) depends upon the onset and grade of cancer-associated anemia, the degree of iron insufficiency, the likelihood of oral absorption, the presence of gastrointestinal comorbidities, and the potential for drug-drug interactions. ESA therapy should not precede treatment of iron deficiency because ESAs will be less effective and may exacerbate thrombocytosis.41 Importantly, recent data in patients without active malignancy suggest that thrombocytosis related to iron-deficiency anemia may increase the risk of thrombosis by nearly twofold compared to those with IDA alone.42 Although oral iron is less expensive, data are divided regarding its effectiveness in treating FIDA34,43 or anemia of inflammation. Two prospective studies in patients with cancer-associated anemia failed to show superiority of IV iron compared with oral iron for improving Hb.43,44 In 1 study, the authors hypothesized that oral iron bioavailability may have improved from tumor response to ANT, although inflammatory markers were not concordant. Furthermore, hepcidin has been shown to be temporarily elevated after ingestion of oral iron,45 but there is no clinical evidence that oral iron prescribed on an alternate-day vs once- or twice-per-day schedule46 improves efficacy in cancer-associated anemia and may be inferior because of elevated hepcidin levels at baseline in patients with cancer. Ultimately, patients may not tolerate oral iron in any form, and even if it is tolerated, the patient may have profound iron deficiency or ongoing losses such that oral iron is incapable of repleting iron stores.
Case 1
In the face of ongoing blood loss and AIDA, IV iron is the preferred method of correction; our 3 favored products are low molecular weight (LMW) iron dextran (500-2000 mg/dose),34,47,48 ferric carboxymaltose (FCM; 750 mg/dose),49,50 and iron sucrose (200-300 mg/dose) because these products have been extensively studied in cancer patients,51 and LMW iron dextran and FCM can be given in larger doses to correct AIDA in the shortest timeframe. Iron repletion dosage calculations, such as the Ganzoni, InFed, and Hanson formulas, have not been validated in the cancer population.52 Depending upon patient weight, iron doses of 1000 to 1500 mg replenish body stores,53 and often increase Hb by 2 to 3 g/dL in the absence of ongoing blood loss. We minimize and, when possible, synchronize blood draws to avoid iatrogenic anemia.
IV iron clinical trial data
As of August 2020, 8 clinical trials have been published using IV iron monotherapy to treat chemotherapy-induced anemia (CIA). A summary of these trials indicates that IV iron either improved Hb levels, reduced RBC transfusions, or both.51,54 IV iron can be used as monotherapy or with ESAs to treat CIA. Consensus guidelines from several organizations have affirmed the use of IV iron to treat CIA,55-57 although the use of IV iron monotherapy has not yet been recommended by all groups.
Risks of IV iron therapy
Table 3 summarizes current IV iron products in use for cancer-associated anemia. All have an iron core coated by a carbohydrate shell; the chemical nature of this carbohydrate is the distinctive feature of each product. Acute toxicity in the form of infusion reactions is possible and is thought to result from complement activation leading to the generation of anaphylatoxin (eg, C5a58 ) rather than immunoglobulin E (IgE)–mediated mast cell activation. Reactions may include vasodilation, nausea, flushing, urticaria, and wheezing. Sternal chest pain and lower back pain may be a consequence of erythropoiesis and marrow expansion. Although older products such as Dexferrum were associated with substantial toxicity, including anaphylaxis,59 a systematic review and meta-analysis of 103 IV iron trials using 7 newer products concluded that minor infusion reaction rates occurred in ∼1 of every 200 infusions; major infusion reaction rates were ∼1 in 200 000 infusions.60 There were several key safety conclusions: there was no increase in serious adverse events (AEs) compared with control (no iron, placebo, oral iron); there was no increased risk of serious infections; ferric gluconate, but not other IV iron products, was associated with serious infusion reactions; and no deaths or anaphylaxis were attributable to IV iron.60 Of note, because limiting iron to certain virally infected cells (eg, HIV, cytomegalovirus, hepatitis C virus, and herpes simplex virus 1) has been shown to curtail viral replication,61 caution is encouraged before administering IV iron to patients with cancer who are co-infected by these viruses. Clinicians should be aware that ferumoxytol, with its unique superparamagnetic properties, can be used as a magnetic resonance imaging (MRI) contrast agent and may alter MRI images up to 3 months after infusion, potentially interfering with interpretation.62 Thus, if patients receive ferumoxytol, radiologists should be made aware of this so they can appropriately interpret MRI images. Although early data are encouraging, there are no long-term safety data evaluating the effects of any IV iron product on cancer-related mortality.
How should IV iron AEs be managed?
Because AEs in response to IV iron are not classic mast cell–mediated allergic reactions, antihistamines such as diphenhydramine are not useful, and this drug itself may cause AEs.63 Rampton et al59 have summarized a treatment strategy for IV iron infusion reactions. Mild reactions are treated by stopping the infusion and resuming it at a lower infusion rate; moderate to severe reactions are treated by stopping the infusion, treating with IV fluids and corticosteroids, and escalating care, as needed.59
How should this patient be monitored after IV iron therapy?
We monitor monthly complete blood counts and iron studies (ferritin, TSAT)37 ; if ferric carboxymaltose is used, phosphate levels are monitored, and the patient is treated with foods rich in phosphorous for mild deficiency or prescription phosphate if the patient is severely deficient. Hypophosphatemia typically resolves within 3 to 6 weeks. We repeat IV iron treatment if AIDA returns or if the ferritin plateaus and declines to <100 ng/mL and TSAT remains <20% (Figure 3).
Is IV iron monotherapy useful in FIDA?
Few adequately powered trials have prospectively evaluated the safety and efficacy of parenteral iron monotherapy for patients with CIA or cancer-associated anemia43,50,54 plus FID, and only 2 studies50,54 have correlated response with hepcidin concentrations. In the only placebo-controlled study conducted to date,50 anemic patients with cancer (Hb, 8-11 g/dL) and FIDA (study defined as TSAT ≤35%, ferritin 100-800 ng/mL) who were receiving ANT were randomly assigned to 2 infusions of either FCM 15 mg/kg (up to 750 mg per dose; 1500 mg total) or placebo, administered 1 week apart. The primary end point of Hb maintenance within 0.5 g/dL of baseline favored IV iron at 18 weeks (achieved by 55% vs 40% of patients, respectively). The median time to Hb decrease of more than 0.5 g/dL was 127 days (18.1 weeks) in the FCM arm compared with 43 days (6.1 weeks) in the placebo arm. The difference of 12 weeks between the 2 groups was statistically significant (P = .0063). Venous thromboembolism (VTE) was not increased in the study arm. Fatal AEs were similar in the FCM group (12.3%) and placebo group (11%); none were attributable to study treatment. The percentage of patients with an Hb increase of ≥1 g/dL without receiving a non-study intervention was significantly greater in the FCM arm (71%) than the placebo arm (54%; P = .01). The mean baseline hepcidin concentration was 107 ng/mL, which corroborates findings from another study using similar cumulative iron doses plus ESA therapy,64 in which the response rate was 92% for patients with baseline hepcidin concentrations of ≤64.3 ng/mL; the response rate was 69% when baseline hepcidin exceeded 64.3 ng/mL. In another study that used FCM monotherapy for CIA, a Hb response was seen in 95% of patients with a baseline hepcidin ≤34.1 ng/mL.54 Although hepcidin assays seem promising in predicting response to IV iron monotherapy, these tests are not widely available, and the threshold for response is not adequately defined.
Optimal dose and timing of IV iron are unknown
Current data do not demonstrate the superiority of large up-front doses (termed “total dose infusion”) when compared with smaller, intermittent doses.34,43,52 Although there are currently no drug label or payer restrictions to using IV iron in treating patients with cancer in regards to stage, curability, or cancer treatment modality, as with any treatment, IV iron bears the burden of proof. Importantly, prospective studies that evaluated the safety and optimal dose of IV iron have not extended beyond 18 weeks. When possible, we avoid administering iron during periods of severe neutropenia. Recent data suggest no increased risk of VTE, and it is presently unknown whether first correcting IDA protects against cancer- or ESA-related VTE.41
Case 2
Patient 2 is a 76-year-old man with a history of autoimmune disease and newly diagnosed metastatic non–small cell lung cancer. Molecular mutation testing is negative. Baseline iron studies showed serum ferritin of 535 ng/mL and TSAT of 23%. He began receiving carboplatin plus paclitaxel once every 3 weeks. After 2 cycles, the Hb decreased from 12.2 g/dL (baseline) to 9.9 g/dL. A review of concomitant medications is unrevealing. CHr is within normal limits, and reticulocytes are inappropriately low for the degree of anemia. After 2 more cycles, the Hb declines to 7.4 g/dL and the patient receives a 2-unit RBC transfusion. How should this patient's anemia be treated?
Are ESAs still used for cancer-associated anemia?
The treatment of cancer-associated anemia with ESAs began in the late 1990s. A summary indicates that ESAs reduce RBC transfusions, and in some cases, ESA dosing may be lowered by the addition of IV iron.51 As of August 2020, 7 clinical trials have been published as full articles (no abstract-only citations) that studied CIA treated with ESAs plus IV iron. However, because the risks of ESAs are well known (including VTE65 ) and potentially decrease cancer patient survival,66-68 we reserve ESAs for patients with non-myeloid malignancies and iron-refractory anemia that require frequent transfusions with packed RBCs (PRBCs), or for patients with decreased QOL after progressive cycles of palliative myelosuppressive ANT.55 Despite their labeled indications and guideline recommendations, it is important to note that no prospective clinical study stratified patients with cancer who were receiving ESAs according to whether ANT was administered with curative or palliative intent. To prevent unnecessary clinic visits, and when payers permit it, we obtain ESAs for home use that use prefilled syringes available in fixed doses (Table 3). When payers request them, biosimilar agents are acceptable.69,70 For ESA monitoring and dosage modifications, we refer the reader to the recently published American Society of Clinical Oncology/American Society of Hematology (ASCO/ASH) guidelines on management of cancer-associated anemia with ESAs.71 We do not measure a baseline serum EPO level in patients who do not have myelodysplastic syndrome because it has been demonstrated that it does not predict response.72 A US Food and Drug Administration–mandated Risk Evaluation and Mitigation Strategies program was ended in 201773 ; however, text that was recently added to ESA drug labels states that ESAs are “not indicated for patients with cancer receiving myelosuppressive chemotherapy in whom the anemia can be managed by transfusion.”74 Literal interpretation of this update seemingly disqualifies the vast majority of patients from receiving ESA therapy, including our patient in case 2. However, after informing patient 2 of the benefits and risks, including counseling him on the signs and symptoms of VTE, our preference is to offer ESA therapy to minimize transfusions and potentially improve his QOL. The results of recently published pharmacovigilance trials conducted in certain cancer subtypes, including lung cancer, have demonstrating that ESAs may be safely used in carefully selected patient populations such as the one presented in case 2.75-78
ESA-induced tumor progression
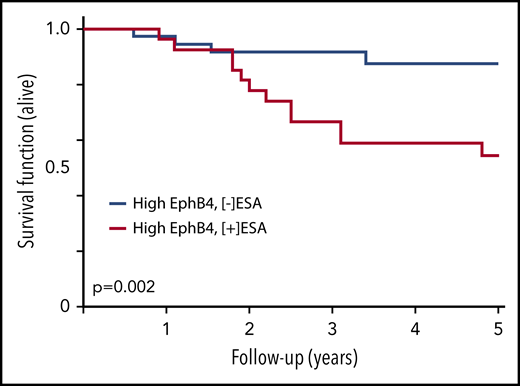
Investigation of the mechanisms responsible for poorer ESA-related outcomes gave rise to myriad hypotheses79 including tumor-expressing EPO receptors,78 hyperviscosity,80 and VTE events owing to increasing hematocrit. After determining that EPO receptor expression did not correlate with decreased survival, further investigation led to the ephrin-B2 ligand and ephrin-B4 receptor hypothesis.81 The membrane-bound ephrin-B2 ligand and its cognate receptor EphB4 are expressed in numerous tumor-specific blood vessels and are upregulated in various malignancies, including breast cancer.82 Each has been shown to be inducible to increase angiogenesis and may participate in tumor neovascularization. Because recombinant human erythropoietin (rhEPO) competes with ephrin-B2 for binding to EphB4 in a dose-dependent manner,81 disease progression may be mediated through rhEPO-induced phosphorylation of EphB4, Src, and STAT3 activation independent of the EPO receptor. The ability to discern whether tumors express EphB4 may enable identification of patients at low vs high risk for disease progression when using ESAs (Figure 4).
Survival of breast cancer patients correlates with expression of tumor tissue EphB4 and ESA therapy. Tissue samples from a cohort of patients with breast cancer were stained for EphB4. These patients had similar demographic and tumor prognostic features. The patient subgroup with high EphB4 expression was analyzed for survival based upon patients being exposed to ESA or not. Patients with high EphB4 expression who received ESA therapy had a shorter survival than those who did not receive ESA therapy. Adapted from Pradeep et al,81 with permission.
Survival of breast cancer patients correlates with expression of tumor tissue EphB4 and ESA therapy. Tissue samples from a cohort of patients with breast cancer were stained for EphB4. These patients had similar demographic and tumor prognostic features. The patient subgroup with high EphB4 expression was analyzed for survival based upon patients being exposed to ESA or not. Patients with high EphB4 expression who received ESA therapy had a shorter survival than those who did not receive ESA therapy. Adapted from Pradeep et al,81 with permission.
Transfusions
RBC transfusions in cancer patients have been linked to an increased risk of thrombosis,83 cancer recurrence, and decreased survival.83-85 Other known risks include pathogen transmission, transfusion reactions, iron or volume overload, and alloimmunization.66 RBC transfusions are associated with a 10-fold greater risk of major morbidity than IV iron (1 in 21 413 for RBCs vs ∼1 in 200 000 for current IV iron products).60 Thus, it is ultimately up to the clinician to consider clinical indicators that show a need for transfusion rather than laboratory abnormalities alone. ASH recently advocated for the continued use of transfusions for patients on hospice as a QOL measure when concerns regarding transfusion-related risks have been de-emphasized.86 However, reimbursement of transfusions in the hospice setting may be problematic, and patients must make the difficult choice between benefits of hospice vs continued transfusion support. Although each unit of PRBCs contains ∼250 mg of iron, that iron is not immediately bioavailable because transfused RBCs live for roughly 90 days. RBC transfusion, which takes approximately 1 to 2 hours per unit to administer, remains an option for treating grade 2 to 4 cancer-associated anemia when other therapies fail, but it may be refused by those with religious proscriptions. In these instances, ESAs may be offered.87,88
Case 3
Patient 3 is an 82-year-old diabetic man who presents with weight loss, dyspnea, and anemia. He is diagnosed with prostate cancer metastatic to the bone and lung; after androgen deprivation therapy fails, he is deemed castrate refractory and is offered enzalutamide, an androgen receptor inhibitor. Laboratory results showed Hb 10.1 g/dL, hematocrit 28%, MCV 81 fL, and platelet count 122 000/μL; iron studies showed ferritin 592 ng/mL and TSAT 18%. Serum creatinine was 1.2 mg/dL, and his weight was 73 kg.
Patients receiving therapy aimed at continually suppressing cancer growth such as anti-hormonal agents or tyrosine kinase inhibitors may experience cancer-associated anemia indefinitely. Ferritin is elevated because of widespread cancer and inflammation. The borderline low TSAT indicates FIDA. Cancer-associated anemia can be especially difficult to address when the Hb is 10.1 to 11.9 g/dL, a condition not low enough to use PRBCs and not severe enough to qualify for ESA therapy. Moreover, ESAs are not indicated for patients with cancer who are receiving hormonal therapy. Androgen therapy is avoided in patients with androgen-responsive tumors.89 Myelosuppressive chemotherapy is the only ANT that qualifies the cancer patient for an ESA; all other anemias must be considered cancer-associated anemia and treated by other means, such as IV iron monotherapy or transfusion when indicated. Enzalutamide does not constitute myelosuppressive chemotherapy; therefore, using ESAs under this setting is contraindicated. It may be tempting to diagnose this patient with stage III chronic kidney disease (glomerular filtration rate <60 mL/min/1.73 m2) which would allow for future use of ESAs. We do not recommend this approach because of the risk of unintended consequences, such as the potential for harm induced by ESAs given to patients with cancer who have not been treated with myelosuppressive ANT.90-92 IV iron monotherapy may provide a marginal Hb increment. If hormonal therapy fails and myelosuppressive chemotherapy is considered, ESA use would then become an option.
Case 4
Patient 4 is a 63-year-old woman diagnosed with estrogen receptor–positive, human epidermal growth factor receptor 2 (HER2)–negative metastatic breast cancer. Her baseline Hb is 13.8 g/dL (MCV 89 fL), but it declines to 7.9 g/dL (MCV 107 fL) after 4 cycles of the oral cyclin-dependent kinase (CDK) inhibitor of CDK4 and CDK6 palbociclib and the parenteral anti-estrogen agent fulvestrant. Her reticulocyte response is inadequate at 62 000 cells per µL. She reports taking ibuprofen 800 mg by mouth 3 times per day as needed for headache and bone pain. What are her anemia therapy options?
By using Table 2, we identify palbociclib as a potential drug-induced cause of decreased RBC production and ibuprofen as potentially contributing to blood loss. Despite macrocytosis, iron studies are performed, and they reveal a transferrin saturation of 6% and a serum ferritin of 23 ng/mL, presumably from ibuprofen-related occult bleeding. Examination of a peripheral smear suggests dysplastic changes. Literature review of CDK4-/CDK6-induced anemia reveals the potential for macrocytic dysplastic changes.93 Oral iron is avoided, given the severity of her anemia and the desire for rapid Hb correction in this patient with AIDA. Parenteral LMW iron dextran at an initial dose of 1 g is given. Her Hb increases to 11.3 g/dL 4 months later. Although unlikely to be deficient, folate, vitamin B12, and thyroid function tests are checked for completeness, and all are within normal limits.8
Adjunctive therapies for cancer-associated anemia
Androgen therapy drugs such as testosterone and danazol have been used successfully to treat premalignant anemias with immune-mediated causes (eg, pure red cell aplasia, aplastic anemia94 ) and telomeropathies.95 Androgens work by downregulating hepatic hepcidin messenger RNA through bone morphogenetic protein/suppressor of mothers against decapentaplegic (BMP/SMAD) signaling and upregulating renal EPO messenger RNA expression.96 Although androgens are unlikely candidates to fully correct cancer-associated anemia in patients with advanced-stage solid tumors, they have been proven effective in patients with early-stage myelofibrosis.97
Future directions
Ascorbic acid
The impact of ascorbic acid (AA; vitamin C) on cancer-associated anemia has yet to be fully explored. In patients with iron-refractory iron-deficient anemia (IRIDA), a genetic mutation in transmembrane protease serine-6 (TMPRSS6) produces an abnormal matriptase-2 protease. Matriptase-2 is responsible, in part, for regulating hepcidin transcription by cleaving hemojuvelin, a co-receptor for BMP/SMAD signaling. Despite parenteral iron supplementation designed to circumvent oral iron absorption blocked by hepcidin in the gut, iron still becomes sequestered in macrophages in patients with IRIDA, a condition which may mimic both anemia of inflammation and FIDA. Interestingly, Sourabh et al98 found that patients with IRIDA who were given AA plus oral iron supplementation experienced an Hb increase of 2 g/dL in 6 (85%) of 7 patients. Chiu et al99 noted that AA downregulated hepcidin expression in liver HepG2 cells, but the mechanisms behind the ability of AA to promote iron mobilization have not been fully characterized. Further evidence supporting the role of AA in mobilizing iron can be seen in anemic hemodialysis-dependent patients with chronic kidney disease who have elevated ferritin and are hyporesponsive to ESAs100,101 and in β-thalassemia major populations.102 Although AA supplementation has the potential to benefit patients with cancer and FIDA, there have been no randomized trials.
Prolyl hydroxylase domain inhibitors
Roxadustat, an oral inhibitor of prolyl hydroxylase, was recently shown to effectively increase HIF-2⍺, leading to a 30-fold increase in EPO concentration in patients with or without hemodialysis-dependent chronic kidney disease.103,104 However, increasing endogenous EPO production may be analogous to administration of exogenous EPO (eg, ESAs), thus potentially limiting the use of prolyl hydroxylase domain inhibitors for cancer-associated anemia because of the risk of tumor progression (Figure 2E).
Activin traps
Luspatercept (ACE-536), an agent designed to promote terminal differentiation of erythropoiesis, is currently being studied for chronic kidney disease, and myelofibrosis, and has recently been approved for patients with β-thalassemia and certain very low- to intermediate-risk myelodysplastic syndrome subtypes.105 Erythroid cells express a type IIB activin receptor that binds members of transforming growth factor-β (TGF-β). TGF-β molecules are thought to reduce terminal erythroid differentiation in these disorders. Luspatercept, an Fc fragment of IgG1 fused to activin receptor, may bind to TGF-β ligands (acting as an activin receptor ligand trap) to prevent them from binding to erythroid progenitors, thereby reducing SMAD2/3 signaling and allowing erythroid differentiation. Luspatercept works independently of EPO and iron but has not been studied for the general treatment of cancer-associated anemia.
Hepcidin antagonists
In the first clinical trial using a monoclonal antibody against hepcidin (LY2787106), the effect on iron and cancer-associated anemia was assessed in patients with cancer who were receiving myelosuppressive chemotherapy.106 Although a significant increase in serum iron and TSAT occurred a few hours after hepcidin infusion, these values returned to baseline within 1 week; ferritin, Hb, and reticulocyte count at the maximum studied dose were not affected. Moreover, oral iron supplementation did not affect response. Although the results of this hepcidin neutralization study seem to be discouraging, the results should be interpreted with caution given the aforementioned complexities of cancer-associated anemia.
Bone morphogenetic protein antagonists to BMP-2 or BMP-6
Bone morphogenetic protein-2 (BMP-2) and BMP-6 are synthesized in liver sinusoidal endothelial cells and bind to BMP receptors in hepatocytes to initiate SMAD signaling, which induces transcription of hepcidin via the HAMP gene.17 Hemojuvelin controls the acute hypoferremic response by regulating the BMP-6 receptor.19 Diminishing the activity of BMP-2, ΒMP-6, or hemojuvelin may decrease hepcidin transcription. However, there are complex feedback mechanisms,107-110 and additional work is required to determine the safety and utility of augmenting this pathway.
Unanswered questions
Given the intricate mechanisms driving cancer-associated anemia, it is clear that no single drug will be both safe and effective for all patients. Clinical trials aimed at correcting cancer-associated anemia should enroll patients with similar underlying comorbidities and concomitant medications to better define populations likely to benefit from a single intervention. Moreover, clinicians may attribute fatigue solely to anemia, but causes of fatigue in patients with cancer are numerous and may not resolve through correction of anemia alone. Novel EPO receptor agonists that leave EphB4 unaffected may prove safer from a disease progression standpoint.111 Baseline hepcidin levels seem to strongly correlate with response to IV iron, but it is uncertain when this assay will be routinely available.
Conclusion
Despite the development of targeted agents, cancer-associated anemia remains prevalent among patients with cancer. Strategies aimed at minimizing cancer-associated anemia should first identify all contributing causes before treatment is considered. Contributing factors often change as patients move from prediagnosis, to treatment, to remission, and continual evaluation is required to identify optimal treatment of persistent anemia. Identification of EphB4 negativity or ESAs without EphB4 cross-reactivity may allow for safer use of agents that work by increasing EPO synthesis. Iron mobilization remains a continued area of interest, and attempts at targeting hepcidin expression require further characterization before the optimal dose and timing of IV iron and novel therapies exploiting this pathway can be deemed both safe and effective. Long-term data evaluating the impact of IV iron therapy on survival in cancer-associated anemia remains a priority.
Acknowledgments
The authors acknowledge the work by investigators involved in basic science and translational and clinical research over the past 30 years, which has greatly increased our understanding of cancer-associated anemia.
Authorship
Contribution: J.A.G. and G.M.R. contributed equally to the original design, draft, and revisions of this manuscript and approved the final version.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Jeffrey A. Gilreath, Department of Pharmacotherapy, University of Utah College of Pharmacy, Huntsman Cancer Institute, 1950 Circle of Hope, Salt Lake City, UT 84112; e-mail: jeffrey.gilreath@hci.utah.edu.
REFERENCES
Author notes
J.A.G. and G.M.R. contributed equally to this work.

![Pathophysiology of cancer-associated anemia. (A) EPO transcription is dependent upon HIF-2⍺, which under normoxic (physiologic oxygenation) conditions is controlled by proteasomal degradation of HIF-2⍺ through a ubiquitination (Ub) process mediated by the von Hippel-Lindau (vHL) protein. With hypoxia, prolyl hydroxylases (PHD) are unable to hydroxylate proline residues on HIF-2α required for vHL binding. This permits HIF-2⍺ to accumulate, dimerize with HIF-1β and bind the EPO promoter region of DNA in renal EPO-producing cells. Secreted EPO migrates to the bone marrow, and upon binding to the EPO receptor located on early erythroid cells (colony forming units-erythroid and proerythroblasts), induces dimerization and phosphorylation of its Janus kinase receptor, leading to erythroblast proliferation.6 Erythrocyte production is closely regulated by the concentration of transferrin-bound iron and may also be diminished by certain anti-neoplastic agents, radiotherapy, or cancer itself.14,15 Upon division, basophilic and polychromatophilic erythroblasts are capable of secreting erythroferrone (ERFE).16 (B) Regulation of iron metabolism involves several rate-limiting steps affecting RBC development. The key regulator of iron metabolism is hepcidin, a small peptide that inhibits iron flux, in both the gastrointestinal tract and splenic macrophages. EPO-dependent induction of ERFE produced by erythroid precursors during stress erythropoiesis functions to suppress hepcidin transcription via bone morphogenetic proteins BMP-5, -6, and -7 to allow for rapid recovery from blood loss.16-18 Hepcidin production is also regulated by iron, with iron excess increasing hepcidin expression (resulting in hypoferremia), and iron deficiency decreasing hepcidin expression.19,20 Hepcidin production is further regulated by inflammation, with cytokines such as interleukin-6 increasing hepcidin synthesis via STAT3 upregulation.21,22 Iron-dependent induction of hepcidin requires hemojuvelin (HJV), a BMP co-receptor, also found on the hepatocyte.19 When serum iron concentrations rise and with the help of HJV, BMP-6 and to some extent BMP-2, bind to their cognate receptor to activate HAMP gene (encodes hepcidin) transcription via the SMAD pathway.19 HFE (high iron) and TFR2 also play a role in BMP signaling.23-25 Hepcidin transcription is reduced when the transmembrane serine protease TMPRSS6 (also known as matriptase-2) cleaves HJV to deactivate the BMP receptor (BMPR). (C) Dietary iron, once reduced (Fe2+), is absorbed via the divalent metal transporter 1 (DMT1) on the apical surface of the intestinal lumen.22 However, once inside this cell, hepcidin, located on the opposite basolateral surface, regulates iron egress into the bloodstream. Hephaestin (not shown) oxidizes Fe2+ to Fe3+ which is required by transferrin for iron docking.22 Inhibition of iron flux occurs when hepcidin binds to ferroportin of gastrointestinal cells and macrophages, leading to ferroportin internalization and degradation.26 (D) Increased levels of hepcidin block mobilization of iron from storage sites, including macrophages in the red pulp of the spleen. (E) Exogenous rhEPO or drug-induced (eg, roxadustat, a prolyl hydroxylase inhibitor [PHDI]) endogenously-produced EPO may bind to the ephrin-B4 receptor on tumor tissue to promote tumor growth. Professional illustration by Patrick Lane, ScEYEnce Studios.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/136/7/10.1182_blood.2019004017/3/m_bloodbld2019004017cf2.png?Expires=1770779444&Signature=K0a6AJIaqVApzWEgAj5Zbn~SEQJ19llHs4sGfTetLoXlJw4D32J7u3lKXY1uo7I7fpiAShmNjvrtsWpOvPBKqtsGFcBfWQgsbw8aBmpUi7opCsp1-7bTcQVVTJXHrPncTh8PlaWnG92IYPPfZPFlAD1bXclQ8toX~mvqT2rPPxwTzOMSErmA9B6BNjWo-wAPs6zptazd2ITXcyrZWsBk92ged7fmQ5EpXIGW2D~FoYg2loL3cTePkxyCRtChlm1LcdaQIXBE6cIJX6OuY8x0zmuO77I0DUdds2erlRCEiuYFRtsW2lDEWAI9r5-fiqjhOAwe0xaXDJkETZnv2O-YAA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)