Abstract
Venous thromboembolism (VTE) is associated with significant mortality and morbidity in patients with cancer. Therefore, tailoring anticoagulation is of utmost importance to decrease the risk of recurrent VTE while minimizing the risk of bleeding. Direct oral anticoagulants have been recently compared with low-molecular-weight heparin for the management of acute cancer-associated thrombosis. Although direct oral anticoagulants are a welcome addition, clinicians need to incorporate clinical characteristics, drug–drug interactions, and patient preference in decision making.
Introduction
Venous thromboembolism (VTE) is a common complication among patients with cancer.1 Although the type and stage of the underlying tumor itself can be an important risk factor for VTE, additional features related to patient’s characteristics (eg, previous VTE, obesity), anticancer treatment (eg, hospitalization, surgery and chemotherapy), and biomarkers have also been shown to increase the risk further.1 The overall incidence of VTE in patients with active cancer is high and reported to be 5.8 (95% confidence intervals [CI], 5.7-6.0) per 100 person-years.2 Despite this high risk of VTE complications, there is low awareness of its importance among patients, which often leads to significant delays in diagnosis and prompt initiation of anticoagulation in those with confirmed events.3-5
VTE is associated with significant mortality, morbidity, and health care costs in patients with cancer. Thromboembolism (VTE and arterial thromboses) has been reported as the second leading cause of death among patients with cancer.6 In patients with cancer-associated thrombosis, the overall mortality rate is 67.7 (95% CI, 65.9-69.7) per 100 person-years with most deaths (>60%) occurring within the first year following the VTE diagnosis.2 Hence, rapid initiation of therapeutic doses of an anticoagulant is the cornerstone of the management of VTE in patients with cancer but anticoagulation might also be associated with significant morbidity. Patients with cancer are more likely to have recurrent VTE despite anticoagulation and to suffer major bleeding complications compared with patients without cancer.7 The rate of recurrent VTE among patients with cancer is 9.6 (95% CI, 8.8-10.4) per 100-person years, with a 12-month cumulative incidence of major bleeding at 12.4% (95% CI, 6.5-18.2).2,7 The case fatality rate of recurrent VTE and major bleeding are 14.8% (95% CI, 6.6-30.1) and 8.9% (95% CI, 3.5-21.1) in this patient population.8 Both recurrent VTE and bleeding complications are also associated with an important decrease in the quality of life for these patients.9 Furthermore, recurrent VTE and major bleeding complications are associated with significant health care resource utilization (eg, hospitalization) and costs.10 Therefore, balancing the need for anticoagulation with decreasing the potential anticoagulant-related complications remains a major challenge. Hence, tailoring anticoagulation with the optimal agents is of utmost importance to decrease the risk of recurrent VTE while minimizing the risk of bleeding and reduce morbidity and health care costs in this patient population.
Acute treatment of cancer-associated thrombosis
Low-molecular-weight heparin (LMWH) monotherapy has been the standard of care for the treatment of acute cancer-associated thrombosis for many years.11-13 In patients with cancer and VTE, LMWH is associated with a lower risk of recurrent VTE (risk ratio [RR], 0.58; 95% CI, 0.43-0.77) without a significant increase in the rate of major bleeding complications (RR, 1.09; 95% CI, 0.55-2.12)14 when compared with vitamin K antagonists (VKA). LMWHs have several advantages over VKAs, such as fewer drug–drug interactions and dependable administration of the anticoagulant effect via a parenteral route. However, this need for daily injections and associated costs are perceived as burdensome to some patients and their physicians, leading to significantly lower persistence, shorter duration of treatment, and more switching to an oral anticoagulant compared with VKAs.15
Direct oral anticoagulants (DOAC) may represent a convenient and effective alternative to VKA and parenteral LMWH for the treatment of acute cancer-associated thrombosis given that they do not require laboratory monitoring. Three direct Xa inhibitors (apixaban, edoxaban, and rivaroxaban) and 1 direct thrombin inhibitor (dabigatran) are currently indicated for the acute treatment of VTE. DOAC were shown to have a similar efficacy and a lower risk of major bleeding (especially intracranial bleeding) compared with VKA in patients with acute VTE in the general population.16,17 Hence, clinical practice guidelines are now recommending their use as first-line therapy for patients with VTE.12 However, more research comparing DOAC to LMWH was needed for patients with cancer and VTE. Recently, 4 randomized controlled trials (RCT) have compared the use of DOAC to LMWH for the acute treatment of cancer-associated thrombosis (Table 1).
Characteristics and 6-month outcomes of randomized trials comparing DOAC with LMWH for the acute treatment of cancer-associated thrombosis
| . | Hokusai VTE Cancer . | SELECT-D . | ADAM VTE . | Caravaggio . | ||||
|---|---|---|---|---|---|---|---|---|
| . | Edoxaban (n = 522) . | Dalteparin (n = 524) . | Rivaroxaban (n = 203) . | Dalteparin (n = 203) . | Apixaban (n = 150) . | Dalteparin (n = 150) . | Apixaban (n = 576) . | Dalteparin (n = 579) . |
| Males (%) | 277 (53.1) | 263 (50.2) | 116 (57.1) | 98 (48.3) | 72 (48.0) | 73 (48.7) | 292 (50.7) | 276 (47.7) |
| Age, mean (SD) or median (range) | 64.3 (11.0) | 63.7 (11.7) | 67 (22-87) | 67 (34-87) | 64.4 (11.3) | 64 (10.8) | 67.2 (11.3) | 67.2 (10.9) |
| Metastatic disease (%) | 274 (52.5) | 280 (53.4) | 118 (58.1) | 118 (58.1) | 96 (64.0) | 97 (64.7) | 389 (67.5)* | 396 (68.4)* |
| GI cancers (%) | 165 (31.6) | 140 (26.7) | 91 (45.0) | 86 (42.4) | 48 (32.0) | 57 (38.0) | 188 (32.6) | 187 (32.3) |
| Recurrent VTE (%) | 34 (6.5) | 46 (8.8) | 8 (3.9) | 18 (8.8) | 1 (0.7) | 9 (6) | 32 (5.6) | 46 (7.9) |
| Major bleeding (%) | 29 (5.6) | 17 (3.2) | 11 (5.4) | 6 (3.0) | 0 (0) | 2 (1.4) | 22 (3.8) | 23 (4.0) |
| CRNMB (%) | 64 (12.3) | 43 (8.2) | 25 (12.3) | 7 (3.5) | 9 (6.0) | 7 (4.7) | 52 (9.0) | 35 (6.0) |
| . | Hokusai VTE Cancer . | SELECT-D . | ADAM VTE . | Caravaggio . | ||||
|---|---|---|---|---|---|---|---|---|
| . | Edoxaban (n = 522) . | Dalteparin (n = 524) . | Rivaroxaban (n = 203) . | Dalteparin (n = 203) . | Apixaban (n = 150) . | Dalteparin (n = 150) . | Apixaban (n = 576) . | Dalteparin (n = 579) . |
| Males (%) | 277 (53.1) | 263 (50.2) | 116 (57.1) | 98 (48.3) | 72 (48.0) | 73 (48.7) | 292 (50.7) | 276 (47.7) |
| Age, mean (SD) or median (range) | 64.3 (11.0) | 63.7 (11.7) | 67 (22-87) | 67 (34-87) | 64.4 (11.3) | 64 (10.8) | 67.2 (11.3) | 67.2 (10.9) |
| Metastatic disease (%) | 274 (52.5) | 280 (53.4) | 118 (58.1) | 118 (58.1) | 96 (64.0) | 97 (64.7) | 389 (67.5)* | 396 (68.4)* |
| GI cancers (%) | 165 (31.6) | 140 (26.7) | 91 (45.0) | 86 (42.4) | 48 (32.0) | 57 (38.0) | 188 (32.6) | 187 (32.3) |
| Recurrent VTE (%) | 34 (6.5) | 46 (8.8) | 8 (3.9) | 18 (8.8) | 1 (0.7) | 9 (6) | 32 (5.6) | 46 (7.9) |
| Major bleeding (%) | 29 (5.6) | 17 (3.2) | 11 (5.4) | 6 (3.0) | 0 (0) | 2 (1.4) | 22 (3.8) | 23 (4.0) |
| CRNMB (%) | 64 (12.3) | 43 (8.2) | 25 (12.3) | 7 (3.5) | 9 (6.0) | 7 (4.7) | 52 (9.0) | 35 (6.0) |
GI, gastrointestinal; SD, standard deviation.
Recurrent locally advanced or metastatic disease
The Hokusai VTE Cancer trial was an open-label, multicenter, noninferiority RCT comparing edoxaban (LMWH × 5 days followed by edoxaban 60 mg daily) to LMWH (dalteparin 200 IU/kg daily × 1 month then 150 IU/kg daily thereafter) for the treatment of acute VTE in patients with cancer (N = 1046).18 The dose of edoxaban was reduced to 30 mg daily in patients with creatinine clearance of 30 to 50 mL per minute or a body weight ≤60 kg or in those receiving concomitant treatment with potent P-glycoprotein inhibitors. The primary outcome was a composite of first-recurrent VTE or major bleeding over the 12-month follow-up period. Among patients receiving edoxaban, 12.8% developed a recurrent VTE or major bleeding as compared with 13.5% in patients receiving LMWH (hazard ratio [HR], 0.97; 95% CI, 0.70-1.36, P = .006 for noninferiority). There were fewer episodes of recurrent VTE in patients receiving edoxaban (HR, 0.71; 95% CI, 0.48-1.06; P = .09) but more episodes of major bleeding complications (HR, 1.77; 95% CI, 1.03-3.04; P = .04). There was also more clinically relevant nonmajor bleeding (CRNMB) in patients receiving edoxaban (HR, 1.38; 95% CI, 0.98-1.94). The Anticoagulation Therapy in SELECTeD Cancer Patients at Risk of Recurrence of Venous Thromboembolism (SELECT-D) trial was an open-label, multicenter, randomized pilot study that compared rivaroxaban (15 mg twice daily ×21 days followed by 20 mg daily thereafter) to LMWH (dalteparin, as previously described) for the treatment of cancer-associated thrombosis (N = 406).19 The primary outcome was recurrent VTE over a 6-month follow-up period. The cumulative incidence of VTE was lower for patients receiving rivaroxaban (HR, 0.43; 95% CI, 0.19-0.99).19 However, the cumulative incidence of major bleeding was higher with rivaroxaban (HR, 1.83; 95% CI, 0.68-4.96).19 The Apixaban and Dalteparin in Active Malignancy-Associated Venous Thromboembolism (ADAM-VTE) trial was an open-label, multicenter, randomized study that compared apixaban (10 mg twice daily ×7 days followed by 5 mg twice daily thereafter) to LMWH (dalteparin, as previously described) for the acute management of patients with VTE and cancer (N = 300).20 The ADAM-VTE trial differed slightly from the Hokusai VTE Cancer and SELECT-D trials because patients with upper extremity deep vein thrombosis and splanchnic vein thrombosis were also included. The primary outcome was major bleeding complication over a 6-month follow-up period. No patients on apixaban suffered a major bleeding complic0ation compared with 1.4% of patients receiving LMWH.20 More recently, the Caravaggio trial, an open-label, multicenter noninferiority RCT comparing apixaban (apixaban, as previously described) with LMWH (dalteparin, as previously described) for the treatment of acute VTE in patients with cancer (N = 1155), was published.21 The primary outcome was an objectively confirmed recurrent VTE over a follow-up period of 6 months. Among patients receiving apixaban, 5.6% developed a recurrent VTE compared with 7.9% in patients receiving LMWH (HR, 0.63; 95% CI, 0.37-1.07, P < .001 for noninferiority). The cumulative incidence of major bleeding complication was also lower for patients receiving apixaban (HR, 0.82; 95% CI, 0.40-1.69).21 However, the cumulative incidence of CRNMB was higher for patients receiving apixaban (HR, 1.42; 95% CI, 0.88-2.30). Three systematic reviews and meta-analyses combining the results of the 4 RCTs comparing DOACs to LMWH for the acute treatment of cancer-associated thrombosis have been published.22-24 DOACs have lower risk of recurrent VTE at 6 months (RR, 0.66; 95% CI, 0.39-1.13) but higher risk of major bleeding events (RR, 1.32; 95% CI, 0.70-2.47),24 although neither was statistically significant. The overall mortality was similar among patients with cancer-associated thrombosis receiving DOAC or LMWH (HR, 0.99; 95% CI, 0.74-1.32).24 The main cause of mortality was cancer progression, accounting for ∼86% to 88% of deaths in the RCTs.18,21
Clinical practice guidelines have promptly updated their recommendations and included DOACs as a reasonable anticoagulant option for the management of VTE in patients with cancer (Table 2).25,26 The results from the Caravaggio trial makes a compelling argument for adding apixaban as an additional option for the acute treatment of cancer-associated thrombosis (Table 2). Given the lack of direct head-to-head comparison and significant heterogeneity among the clinical trials comparing different DOACs to LMWH, it is currently difficult to recommend 1 DOAC over another.
Recommendations for the acute treatment of cancer-associated thrombosis
| ISTH SSC 201826 . | ASCO 201925 . | ITAC 201937 . | NCCN 202038 . | ESC 201939 . |
|---|---|---|---|---|
| DOACs (edoxaban and rivaroxaban) and LMWH are the preferred agents Choice dependent on the risk of bleeding (LMWH preferred in patients with a high risk of bleeding) and potential for DDIs High risk of bleeding was defined as patients with luminal GI cancers with an intact primary, patients with cancers at risk of bleeding from the GU tract, bladder, or nephrostomy tubes, or patients with active GI mucosal abnormalities such as duodenal ulcers, gastritis, esophagitis, or colitis | Initial anticoagulation (first 5-10 d): LMWH or rivaroxaban preferred Long-term (<6 mo): LMWH, edoxaban, or rivaroxaban (VKAs are acceptable alternatives for long-term therapy if LMWH/DOACs are not available) There is an increase in major bleeding risk with DOACs, particularly observed in GI and potentially GU malignancies. Caution with DOACs is also warranted in other settings with high risk for mucosal bleeding Consider DDI | Initial anticoagulation (first 5-10 d): LMWH, rivaroxaban, or edoxaban following ≥5 d of parenteral anticoagulation Long-term (<6 mo): LMWH or DOACs (to date evidence is only available for edoxaban and rivaroxaban) DOACs should be used with caution in patients with GI cancers, especially upper GI cancers DOACs are recommended for patients with cancer when creatinine clearance is ≥30 mL/min in the absence of strong DDI or GI absorption impairment | Appropriate monotherapy/combined therapy options include: LMWH, edoxaban, apixaban, and rivaroxaban LMWH is preferred for patients with gastric or gastroesophageal lesions because these patients are at increased risk for hemorrhage with DOACs Consider DDI Use anticoagulation with caution in patients with compromised renal or liver function | Long-term for patients with PE and cancer (<6 mo): LMWH are preferred over VKAs Edoxaban or rivaroxaban should be considered as an alternative to LMWH Caution for patients with GI cancer because of the increased risk of bleeding with DOACs |
| ISTH SSC 201826 . | ASCO 201925 . | ITAC 201937 . | NCCN 202038 . | ESC 201939 . |
|---|---|---|---|---|
| DOACs (edoxaban and rivaroxaban) and LMWH are the preferred agents Choice dependent on the risk of bleeding (LMWH preferred in patients with a high risk of bleeding) and potential for DDIs High risk of bleeding was defined as patients with luminal GI cancers with an intact primary, patients with cancers at risk of bleeding from the GU tract, bladder, or nephrostomy tubes, or patients with active GI mucosal abnormalities such as duodenal ulcers, gastritis, esophagitis, or colitis | Initial anticoagulation (first 5-10 d): LMWH or rivaroxaban preferred Long-term (<6 mo): LMWH, edoxaban, or rivaroxaban (VKAs are acceptable alternatives for long-term therapy if LMWH/DOACs are not available) There is an increase in major bleeding risk with DOACs, particularly observed in GI and potentially GU malignancies. Caution with DOACs is also warranted in other settings with high risk for mucosal bleeding Consider DDI | Initial anticoagulation (first 5-10 d): LMWH, rivaroxaban, or edoxaban following ≥5 d of parenteral anticoagulation Long-term (<6 mo): LMWH or DOACs (to date evidence is only available for edoxaban and rivaroxaban) DOACs should be used with caution in patients with GI cancers, especially upper GI cancers DOACs are recommended for patients with cancer when creatinine clearance is ≥30 mL/min in the absence of strong DDI or GI absorption impairment | Appropriate monotherapy/combined therapy options include: LMWH, edoxaban, apixaban, and rivaroxaban LMWH is preferred for patients with gastric or gastroesophageal lesions because these patients are at increased risk for hemorrhage with DOACs Consider DDI Use anticoagulation with caution in patients with compromised renal or liver function | Long-term for patients with PE and cancer (<6 mo): LMWH are preferred over VKAs Edoxaban or rivaroxaban should be considered as an alternative to LMWH Caution for patients with GI cancer because of the increased risk of bleeding with DOACs |
ASCO, American Society of Clinical Oncology; DDI, drug-to-drug interactions; ESC, European Society of Cardiology; GU, genitourinary; ISTH, International Society on Thrombosis and Haemostasis; ITAC, International Initiative on Thrombosis and Cancer; NCCN, National Comprehensive Cancer Network; PE, pulmonary embolism.
Direct oral anticoagulants for all, or just some?
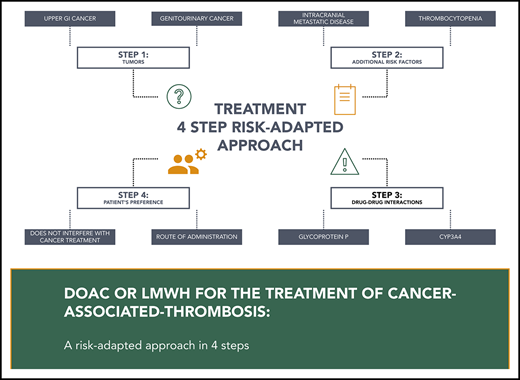
Although DOACs seem to be a convenient, effective, and generally safe alternative to LMWH for the management of acute cancer-associated thrombosis, several factors must be taken into consideration when determining the anticoagulant of choice for a specific patient. Treatment algorithms for patients with cancer and acute VTE have been previously published and suggest incorporating tumor type, risk of bleeding, drug–drug interactions, and patient preference in decision making.27-30
The trials comparing edoxaban and rivaroxaban to LMWH have reported a higher risk of clinically important bleeding episodes in patients receiving DOACs.18,19 The imbalance in bleeding complications in patients receiving DOACs seems to be due to an excess of upper gastrointestinal bleeding occurring mostly in patients with gastrointestinal cancers.31 Interestingly, in the Caravaggio trial, apixaban was not associated with an increased risk of major gastrointestinal bleeding when compared with LMWH (HR, 1.05; 95% CI, 0.44-2.50). The potential explanations for the differences in the risks of major bleeding events remain debatable, including the differences in patient characteristics, the anticoagulant itself, or other factors. There were comparable percentage of enrolled patients with gastrointestinal or upper gastrointestinal cancers as well as with active cancers in both Hokusai VTE Cancer and Caravaggio trials, but the presence of active luminal tumors was not reported in either study. Nonetheless, clinicians should be very careful in using DOACs in patients with upper gastrointestinal cancers or unresected luminal tumors and decide on a case-by-case basis after balancing the potential risks of bleeding with patient preference and values.26
Apixaban, edoxaban, and rivaroxaban are all substrates for P-glycoprotein (P-gp). P-gp is an ATP-dependent efflux pump that plays an important role in the absorption of these agents.32 Hence, drug-to-drug interactions with strong P-gp inducers or inhibitors may lead to a decrease or an increase in drug concentrations, respectively. Apixaban and rivaroxaban are also dependent on cytochrome CYP3A4 for part of their metabolism. Therefore, strong CYP3A4 inducers and inhibitors will also potentially alter their efficacy or safety.33 Although the clinical significance of these drug-to-drug interactions remains unclear, most trials excluded patients with concomitant use of strong inducers or inhibitors of P-gp or CYP3A4. Hence, clinicians should probably prefer LMWH in patients with these drug-to-drug interactions.26
Although DOACs are a welcome addition to the arsenal of therapeutic options for the treatment of cancer-associated thrombosis, more data are needed before they can be used for all patients, especially in challenging cases. For example, there is currently more clinical experience with using LMWH to manage anticoagulated patients with thrombocytopenia.34,35 Similarly, more clinical data on the use of DOACs in patients with extremes of body weights (low and high) would be reassuring.36 Patients with acute leukemia and severe renal insufficiency were also underrepresented in the different RCTs. Last, data on the use of DOACs in patients receiving targeted cancer therapies including antiangiogenic monoclonal antibodies (eg, bevacizumab) and checkpoint inhibitors are desperately needed. Nonetheless, DOACs have certainly changed the landscape of anticoagulation for patients with cancer and VTE and are an effective and safe option for many patients.
Authorship
Contribution: T.-F.W. and M.C. both contributed to the study concept and design, acquisition of data, analysis and interpretation of data, and drafting and revision of the manuscript.
Conflict-of-interest disclosure: M.C. reports research grants from Pfizer, Bristol-Myers Squibb, and Leo Pharma, and consultancy honoraria from Pfizer, Bayer, Sanofi, Servier, and Leo Pharma. M.C. and T.-F.W. participated in the HOKUSAI-VTE trial. T.-F.W. participated in the Caravaggio trial.
Correspondence: Marc Carrier, 501 Smyth Rd, Box 201A, Ottawa, ON, Canada, K1H 8L6; e-mail: mcarrier@toh.ca.