TO THE EDITOR:
Immune thrombocytopenia (ITP) is characterized by autoimmune platelet destruction. Although other autoimmune conditions such as systemic lupus erythematosus (SLE) or multiple sclerosis1,2 have well-described predilections for certain racial and ethnic groups, little is reported on racial and ethnic distributions in ITP. Based on the clinical observation that pediatric ITP is less frequently seen in black individuals, we examined the hypothesis that black children have a lower prevalence of pediatric ITP relative to the general population because of differences in disease biology based on race and ethnicity.
We conducted a retrospective chart review capturing self-reported race and ethnicity data3 for children ages 0 to 18 year treated for ITP at Texas Children’s Hospital (TCH, Houston, TX) and Children’s Hospital of Philadelphia (CHOP, Philadelphia, PA) from January 2015 through July 2019. This review was conducted in accordance with the Declaration of Helsinki. Demographic definitions are shown in supplemental Table 1, available on the Blood Web site. ITP patients were identified by International Classification of Diseases-9/10 codes for immune thrombocytopenia and confirmed by expert review.
Patients with disease resolution within 1 year from diagnosis were defined as having “acute ITP.” Chronic ITP was defined as disease persisting beyond 1 year.4 Disease resolution was defined as: (1) 2 consecutive platelet counts >150 × 109/L at least 1 month apart with mean platelet volume (MPV) ≤11, or (2) single platelet counts >200 × 109/L with normal MPV, or (3) 3 or more consecutive platelet counts >150 × 109/L with an MPV ≤11.5. Platelet counts were considered if obtained off therapy, defined as: 6 weeks since immunoglobulin or Rho(D) immune globulin, 3 weeks since completion of steroid course, ≥2 weeks since immunomodulators and thrombopoietin receptor agonists, or if complete B-cell repopulation was achieved after rituximab.
Medical records were queried for clinical features including primary/secondary ITP, bleeding score, and treatments used. Evans syndrome was defined as 2 autoimmune cytopenias occurring concurrently or sequentially.5 Bleeding scores were defined using the Buchanan and Adix Bleeding Score.6 First-line therapies included immunoglobulin, Rho(D) immune globulin, and/or corticosteroids. Second-line treatments included rituximab, thrombopoietin receptor agonists, mycophenolate mofetil, azathioprine, or splenectomy. The number of unique treatment classes used for each patient was documented; repeated rescue therapy requirements were not recorded.
Demographic data from the Texas Department of State Health Services, Center for Health Statistics were obtained for the Houston metropolitan statistical area, encompassing 9 counties including Harris County, where Houston is located. Population projections for persons aged 0 to 18 in the Houston metropolitan statistical area for 2018 were estimated by applying projections for major components of population change to the baseline data available from the 2010 US census. Race and ethnicity data from the Texas Children’s Cancer Center were also used as comparators.
Patient characteristics were summarized using median with minimum and maximum, or frequency with percentage. Summary statistics were stratified by race and compared using the Wilcoxon rank-sum test, quantile regression, Fisher's exact, or χ2 tests. The proportion of black, non-Hispanic patients in the ITP cohort was compared with the proportion in the corresponding metropolitan area and hospital population using the χ2 test. Kaplan-Meier curves and log-rank tests were used to determine if time to ITP resolution depends on race. Cox proportional hazards regression was used to calculate hazard ratios with 95% confidence interval. A P value <.05 was considered significant.
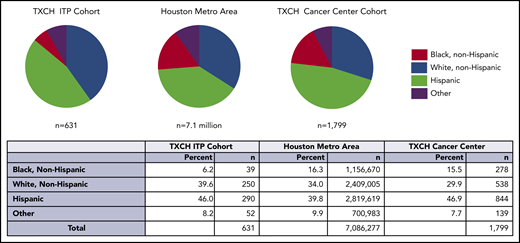
Among 631 evaluable ITP patients seen at the TCH Hematology Center from July 2015 through 2019, the percentage of black, non-Hispanic patients in the ITP cohort was lower than the Houston metropolitan area (6.2% vs 16.3%, P < .001) and TCH Cancer Center (6.2% vs 15.5%, P < .001; Figure 1). There were fewer black ITP patients in both acute and chronic ITP cohorts. Full race and ethnicity breakdown for this cohort and summary statistics are in supplemental Tables 2 and 3.
The proportion of black individuals, indicated in red, was lower in the TXCH ITP cohort compared with the proportion of black individuals in the Houston metropolitan statistical area and TXCH cancer. The difference in the black ITP patient percentage was statistically significant in comparison with both the TXCH cancer center (P < .001) and Houston metropolitan area (P < .001). TXCH, Texas Children’s Hospital.
The proportion of black individuals, indicated in red, was lower in the TXCH ITP cohort compared with the proportion of black individuals in the Houston metropolitan statistical area and TXCH cancer. The difference in the black ITP patient percentage was statistically significant in comparison with both the TXCH cancer center (P < .001) and Houston metropolitan area (P < .001). TXCH, Texas Children’s Hospital.
These findings were validated in 215 ITP patients treated at CHOP. The proportion of black ITP patients at CHOP was significantly lower compared with the CHOP general patient population (5.6% vs 21.6%, P < .001). Although not directly comparable because of differences in how Hispanic individuals were categorized, 43.7% individuals in the Philadelphia County census self-identified as black.7 The percentage of black ITP patients at CHOP was small and not statistically significantly different from that at TCH (5.6% vs 6.2%, P = .750).
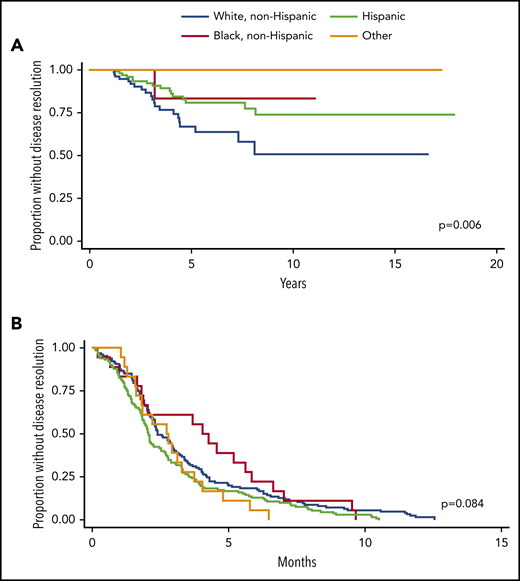
For acute and chronic ITP cohorts, there was no difference among racial groups in gender, age at diagnosis, presence of Evans syndrome, use of second-line therapies, or number of unique treatments used over time. For patients with chronic ITP, the percentage of patients with secondary ITP differed among races (P = .001). Black and Hispanic patients have the highest incidence of secondary ITP (most commonly, SLE for both groups), with 28.6% and 31.5% respectively, whereas 17.5% of white non-Hispanic patients and 0% of patients of other races had secondary ITP. Time to resolution for patients with chronic ITP significantly differs among races (log-rank P = .006, Figure 2).
Time to disease resolution for chronic and acute ITP cohorts by race. (A) Kaplan-Meier curves are shown demonstrating the time to disease resolution for chronic ITP patients of various races. White, non-Hispanic patients (blue) had the shortest time to disease resolution, followed by Hispanic (green) and black, non-Hispanic patients (purple). No patients classified as “other” had disease resolution. The time to disease resolution was different among groups (P = .006). (B) Kaplan-Meier curves are shown demonstrating the time to disease resolution for acute ITP patients of various races. There was no statistically significant difference in time to disease resolution within these groups (P = .084).
Time to disease resolution for chronic and acute ITP cohorts by race. (A) Kaplan-Meier curves are shown demonstrating the time to disease resolution for chronic ITP patients of various races. White, non-Hispanic patients (blue) had the shortest time to disease resolution, followed by Hispanic (green) and black, non-Hispanic patients (purple). No patients classified as “other” had disease resolution. The time to disease resolution was different among groups (P = .006). (B) Kaplan-Meier curves are shown demonstrating the time to disease resolution for acute ITP patients of various races. There was no statistically significant difference in time to disease resolution within these groups (P = .084).
These data of variation in time to disease resolution and secondary ITP incidence by race/ethnicity are novel. The finding that ITP prevalence varies in different racial and ethnic groups is consistent with available literature. One study showed the age of ITP incidence differs between Vietnamese and European cohorts.8 Another cited higher incidence of ITP in Hispanic over white, non-Hispanic patients related to differences in Helicobacter pylori colonization.9
A letter by Terrell et al published in Blood in 2005 identified a low percentage of black ITP patients in 6 other ITP studies where demographics were reported and confirmed this finding in their own population.10-16 In a rebuttal to this study, ITP prevalence did not differ by race in a large cohort of adult, male veterans treated inpatient for ITP.17 However, this study was limited by exclusion of females, who carry increased burden of ITP in adolescent and adult cohorts, focus on hospitalized patients when ITP is typically managed outpatient, and exclusion of those with known autoimmune disorders, which preclude military enlistment.
Our study is limited as a retrospective review and use of self-reported race and ethnicity, which may not accurately reflect genetic ancestry. Further, black, non-Hispanic patients represent a cohort of heterogeneous genetic background.18 Our group is pursuing studies comparing ITP cohorts that are defined by genetic ancestry.
Another study limitation is that differences in medical care utilization related to race are not fully addressed. Darker skin pigmentation may diminish recognition of subtle cutaneous bleeding, leading individuals not to seek care. Socioeconomic factors may also affect access care, especially to subspecialty care. However, the percentage of black, non-Hispanic ITP patients are consistent in our 2 study cohorts and across 7 prior ITP studies.10 Presumably, socioeconomic barriers in different regions would not have nearly identical impacts on access and decrease the proportion of black patients to the same degree.
In conclusion, we have found in 2 large, independent pediatric ITP cohorts that black, non-Hispanic individuals have a lower prevalence of disease than predicted based on racial and ethnic distributions in the general population. In chronic ITP, the incidence of secondary ITP and time to disease resolution differed by race and ethnicity. Providers should have a higher suspicion for alternate diagnoses or secondary causes of ITP when managing black patients with presumed ITP and should consider the diagnostic workup accordingly. Most importantly, these findings suggest there are biologic differences in ITP driven by race and ethnicity and emphasize the need for further genetic studies into the pathogenesis of ITP.
Data sets are available via e-mail to the corresponding author.
The online version of this article contains a data supplement.
Authorship
Contribution: T.O.K. designed the research, collected and analyzed data, and wrote the paper; A.B.G. designed the research, collected and analyzed data, and wrote the paper; M.M.G. and S.E.K. collected and analyzed data and wrote the paper; H.D.R. collected and analyzed data and wrote the paper; K.A.S. designed the research, analyzed data, and wrote the paper; L.A.W. designed the research and collected data; A.A. and A.S.C. collected and analyzed data; M.P.L. collected and analyzed data and wrote the paper; and J.M.D. designed the research, analyzed data and wrote the paper.
Conflict-of-interest disclosure: M.P.L. is a consultant for Dova, Novartis, Shionogi, Amgen, Sysmex, and Rigel and receives research funding from Novartis, Rigel, Sysmex, Astra Zeneca, and the National Institutes of Health. J.M.D. receives research funding from Amgen, Novartis; and is a consultant for Novartis, Agios, and Dova Pharmaceuticals. The remaining authors declare no competing financial interests.
Correspondence: Taylor Olmstead Kim, Baylor College of Medicine, 1102 Bates St, Feigin Building, Suite C1030, Houston, TX 77003; e-mail: teolmste@txch.org.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal