Key Points
A risk stratification model based on patient age and donor type predicts event-free survival after transplant for sickle cell disease.
Variable effectiveness of nontransplant disease-modifying treatments in subpopulations justify alternative donor transplants.
Abstract
We developed a risk score to predict event-free survival (EFS) after allogeneic hematopoietic cell transplantation for sickle cell disease. The study population (n = 1425) was randomly split into training (n = 1070) and validation (n = 355) cohorts. Risk factors were identified and validated via Cox regression models. Two risk factors of 9 evaluated were predictive for EFS: age at transplantation and donor type. On the basis of the training cohort, patients age 12 years or younger with an HLA-matched sibling donor were at the lowest risk with a 3-year EFS of 92% (score, 0). Patients age 13 years or older with an HLA-matched sibling donor or age 12 years or younger with an HLA-matched unrelated donor were at intermediate risk (3-year EFS, 87%; score, 1). All other groups, including patients of any age with a haploidentical relative or HLA-mismatched unrelated donor and patients age 13 years or older with an HLA-matched unrelated donor were high risk (3-year EFS, 57%; score, 2 or 3). These findings were confirmed in the validation cohort. This simple risk score may guide patients with sickle cell disease and hematologists who are considering allogeneic transplantation as a curative treatment relative to other available contemporary treatments.
Introduction
With access to modern health care, survival of children with sickle cell disease (SCD; HbSS and HbSβ0) at age 18 years is 93.9% (95% confidence interval [CI], 90.3%-96.2%) and for those with HbSC and HbSβ+, survival was 98.4% (95% CI, 94.4%-99.5%).1 If a patient survives to age 18 years, the median length of survival for HbSS/HbSβ0/HbSD is 48 years (95% CI, 44.4-58.4 years) and for HbSC/HbSβ+, it is 54.7 years (95% CI, 38.6-62.9 years).2 In the study by DeBaun et al,2 older age had a negative impact on survival, and baseline hemoglobin (Hb) levels were inversely related to the risk of death. Causes of death in adults were predominantly pulmonary hypertension, renal failure, infection, thromboembolic events, and complications of iron overload.3 Hematopoietic cell transplantation is a potentially curative therapy for SCD, because it replaces diseased bone marrow with healthy bone marrow. However, it is limited by donor availability and transplant complications, including graft failure and mortality. In a recent report of 996 patients with SCD who received transplantation, event-free survival (EFS; the probability of being alive with donor engraftment) was highest in children age 12 years or younger and those with an HLA-matched sibling donor.4 For patients without an HLA-matched sibling donor, risks of mortality were greater; the data do not favor one alternative donor over another.4 Given the findings of the study by Eapen et al4 together with increasing numbers of transplantations for SCD, we aimed to provide a robust, simple tool to be used for predicting EFS for allogeneic transplant and for stratifying patients who enter prospective clinical trials of allogeneic transplant.
Study design
In all, 1425 patients with SCD who received a transplant between 2008 and 2017 were randomly split into training (n = 1070) and validation (n = 355) cohorts (supplemental Table 1, available on the Blood Web site). Of those, data for 996 patients included in this analysis were reported earlier.4 The training data set was used to develop a prognostic scoring system for EFS, and the validation data set was used to evaluate the predictive ability of the scoring system.5 EFS was defined as the time from transplantation to graft failure or death assessed throughout the entire follow-up period.4 Covariates predictive of EFS were identified on the basis of the previous publication.4 The scoring system relied on patient-related biologic characteristics assessed as treatment strategies evolved. Risk scores were assigned on the basis of associated hazard ratios and were grouped on the basis of the estimated EFS probabilities into good, intermediate, and high. Harrell’s C statistic was used to assess the predictive ability of the score.6 The level of significance was P value ≤ .05 (two-sided). Analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC).
Results and discussion
Characteristics were similar between the training and validation cohorts, which had a median follow-up of 3 years (range, 0.75-10.4 years), and median age at transplantation was 11 years (range, 1-48 years). Most patients (973 [68%] of 1425) received grafts from an HLA-matched sibling. The remaining patients received grafts from a haploidentical relative (n = 186; 13%) or an HLA-matched (n = 140; 10%) or mismatched unrelated (n = 126; 9%) donor. Transplant conditioning regimen intensity was defined on the basis of previously published criteria.7 Applying these criteria, most patients (972 [68%] of 1425) received myeloablative regimens. Others received reduced-intensity (272; 19%) or nonmyeloablative (181; 13%) regimens.
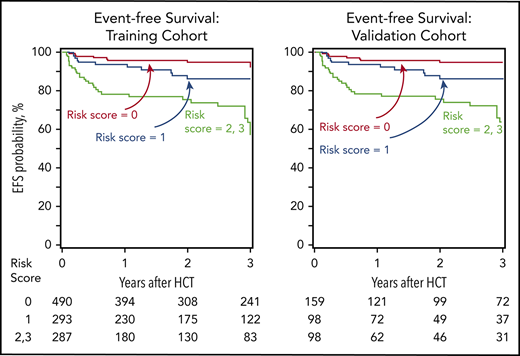
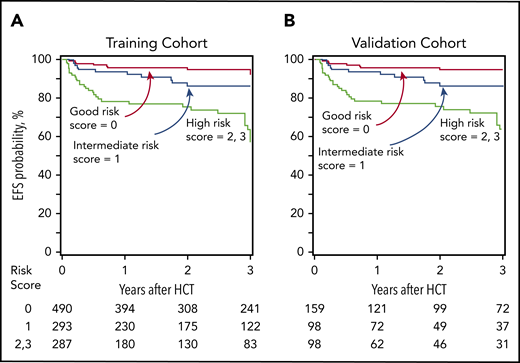
Age at transplantation and type of donor were significantly associated with EFS. A risk score was assigned for patients on the basis of their age and donor type. Patients age 12 years or younger received 0 points, and those age 13 years or older received 1 point. Patients with an HLA-matched sibling donor received 0 points, those with an HLA-matched unrelated donor received 1 point, and those with a haploidentical relative or HLA-mismatched unrelated donor received 2 points. The final risk score was calculated by adding the score associated with age (0, 1) and type of donor (0, 1, 2) as shown in Table 1. A relative risk for graft failure or death (1-EFS) was based on the final risk score, which was classified as good, intermediate, or high (Table 2). The EFS by risk group for the training and validation cohorts is shown in Figure 1A-B. With 3-year EFS >90% for the good-risk group, this outcome favors transplantation when clinically indicated. Although EFS was lower in the intermediate-risk group, transplantation may be curative. However, graft-versus-host disease in children remains a concern (supplemental Table 2).
Risk score based on age at transplantation and type of donor (training cohort)
| Age, y . | Age score . | Type of donor . | Donor score . | Total score . | 3-year probability/incidence % (95% CI) . | ||
|---|---|---|---|---|---|---|---|
| EFS . | Death without graft failure . | Graft failure . | |||||
| ≤12 | 0 | HLA matched sibling | 0 | 0 | 92 (89-94) | 2 (0-4) | 6 (4-9) |
| 0 | HLA mismatched relative | 2 | 2 | 62 (43-76) | 8 (2-19) | 30 (15-47) | |
| 0 | Matched unrelated donor | 1 | 1 | 83 (69-91) | 8 (2-18) | 8 (2-18) | |
| 0 | Mismatched unrelated donor | 2 | 2 | 68 (55-79) | 5 (1-13) | 27 (16-38) | |
| ≥13 | 1 | HLA matched sibling | 0 | 1 | 87 (81-92) | 7 (4-11) | 5 (2-10) |
| 1 | HLA mismatched relative | 2 | 3 | 52 (38-65) | 10 (4-18) | 38 (24-51) | |
| 1 | Matched unrelated donor | 1 | 2 | 50 (34-64) | 29 (17-43) | 21 (10-33) | |
| 1 | Mismatched unrelated donor | 2 | 3 | 49 (31-66) | 23 (9-40) | 28 (13-44) | |
| Age, y . | Age score . | Type of donor . | Donor score . | Total score . | 3-year probability/incidence % (95% CI) . | ||
|---|---|---|---|---|---|---|---|
| EFS . | Death without graft failure . | Graft failure . | |||||
| ≤12 | 0 | HLA matched sibling | 0 | 0 | 92 (89-94) | 2 (0-4) | 6 (4-9) |
| 0 | HLA mismatched relative | 2 | 2 | 62 (43-76) | 8 (2-19) | 30 (15-47) | |
| 0 | Matched unrelated donor | 1 | 1 | 83 (69-91) | 8 (2-18) | 8 (2-18) | |
| 0 | Mismatched unrelated donor | 2 | 2 | 68 (55-79) | 5 (1-13) | 27 (16-38) | |
| ≥13 | 1 | HLA matched sibling | 0 | 1 | 87 (81-92) | 7 (4-11) | 5 (2-10) |
| 1 | HLA mismatched relative | 2 | 3 | 52 (38-65) | 10 (4-18) | 38 (24-51) | |
| 1 | Matched unrelated donor | 1 | 2 | 50 (34-64) | 29 (17-43) | 21 (10-33) | |
| 1 | Mismatched unrelated donor | 2 | 3 | 49 (31-66) | 23 (9-40) | 28 (13-44) | |
Risks for EFS
| Risk group . | Training cohort (Harrell’s C statistic, 0.7134) . | Validation cohort (Harrell’s C statistic, 0.7236) . | ||||
|---|---|---|---|---|---|---|
| No. of events/No. of patients . | Hazard ratio (95% CI) . | P . | No. of events/No. of patients . | Hazard ratio (95% CI) . | P . | |
| Good (score, 0) | 33/490 | 1.00 | <.0001 | 8/159 | 1.00 | <.0001 |
| Intermediate (score, 1) | 31/293 | 1.64 (1.01-2.67) | .0482 | 12/98 | 2.52 (1.03-6.16) | .0431 |
| High (score, 2-3) | 104/287 | 6.73 (4.54-9.96) | <.0001 | 33/98 | 7.71 (3.56-16.71) | <.0001 |
| Risk group . | Training cohort (Harrell’s C statistic, 0.7134) . | Validation cohort (Harrell’s C statistic, 0.7236) . | ||||
|---|---|---|---|---|---|---|
| No. of events/No. of patients . | Hazard ratio (95% CI) . | P . | No. of events/No. of patients . | Hazard ratio (95% CI) . | P . | |
| Good (score, 0) | 33/490 | 1.00 | <.0001 | 8/159 | 1.00 | <.0001 |
| Intermediate (score, 1) | 31/293 | 1.64 (1.01-2.67) | .0482 | 12/98 | 2.52 (1.03-6.16) | .0431 |
| High (score, 2-3) | 104/287 | 6.73 (4.54-9.96) | <.0001 | 33/98 | 7.71 (3.56-16.71) | <.0001 |
EFS. (A) Training cohort. The 3-year EFS was 92% (95% CI, 89%-94%) for good-risk, 87% (95% CI, 82%-91%) for intermediate-risk, and 57% (95% CI, 50%-64%) for high-risk patients. (B) Validation cohort. The 3-year EFS was 95% (95% CI, 89%-98%) for good-risk, 86% (95% CI, 77%-92%) for intermediate-risk, and 63% (95% CI, 51%-72%) for high-risk patients.
EFS. (A) Training cohort. The 3-year EFS was 92% (95% CI, 89%-94%) for good-risk, 87% (95% CI, 82%-91%) for intermediate-risk, and 57% (95% CI, 50%-64%) for high-risk patients. (B) Validation cohort. The 3-year EFS was 95% (95% CI, 89%-98%) for good-risk, 86% (95% CI, 77%-92%) for intermediate-risk, and 63% (95% CI, 51%-72%) for high-risk patients.
An important finding is a 3-year EFS of ∼60% in the high-risk group, which included children age 12 years or younger with a haploidentical relative or HLA-mismatched unrelated donor. With improved access to high-quality health care, the incidence of stroke, vaso-occlusive crisis, and acute chest syndrome has decreased substantially in children with excellent survival through age 18 years.1,8-11 Because responses vary to therapies other than transplantation in patients who require regular blood transfusion for secondary prevention of central nervous system infarction in children at increased risk of stroke,12-14 HLA-mismatched donor transplantation may be preferred. Multiple vaso-occlusive crises or acute chest syndrome in children have not been shown to predict mortality or progressive end-organ damage through childhood and adolescence,8 and high-quality disease-modifying care may be preferred over HLA-mismatched donor transplantation in this population. Graft failure was the predominant cause of treatment failure in children in the high-risk group. Because it has been observed that graft failure is often a late event occurring beyond the second year after transplantation, there is a need for follow up beyond 2 years.
Adolescents and young adults without an HLA-matched sibling also fall into the high-risk group. However, as patients with SCD age, many develop clinical and preclinical manifestations of end-organ damage, which further contributes to morbidity and mortality despite continued use of hydroxyurea.3 In the study by Maitra et al,3 factors associated with higher mortality included increased reticulocyte count, elevated tricuspid valve jet regurgitant velocity, pro-B-type natriuretic peptide, and a low fetal Hb level. Thus, transplantation may be acceptable for those adolescents and young adults seeking a cure, despite the higher risk of graft failure and death. Treatment options continue to evolve, influencing the discussion on risks and benefits of transplant and nontransplant treatment options. Future studies should evaluate the effect of pretransplant clinical and preclinical manifestations of end-organ damage, including cardiopulmonary, hepatic, and renal effects and morbidity (annual hospitalizations for pain, acute chest syndrome, stroke) on transplant outcomes because these data were not available in our population.3,15,16 Studies are also needed to determine the incidence of additional end-organ damage from the transplant itself in patients who receive a transplant compared with similar patients who did not proceed to transplant.
The proposed risk score model does not incorporate treatment regimens because they are expected to evolve and can be tailored to each patient. However, because we previously showed that conditioning intensity has an impact on EFS, we examined EFS in each risk group (supplemental Table 3).4 Unlike patients in the good- and intermediate-risk groups who fared well with transplantation, patients in the high-risk group had a low 3-year EFS regardless of regimen intensity or transplant period, suggesting the relative importance of donor-recipient HLA mismatching. Studies of regimens aimed at overcoming graft failure associated with HLA-mismatched transplantation are ongoing.17-19 Most regimens in our analysis included busulfan at myeloablative doses, but pharmacokinetic data were not available.
In summary, a robust and simple prognostic tool was developed to assess the relative risks of allogeneic hematopoietic cell transplantation for sickle cell disease on the basis of age and donor type, which could objectively aid in decision-making on the use of allogeneic transplantation compared with disease-modifying standard-of-care treatments. Transplantation-associated risks may be acceptable for subpopulations of children, adolescents, and young adults without an HLA-matched sibling, if the risks of graft failure and death are recognized and considered.
For original data, please e-mail Ruta Brazauskas at ruta@mcw.edu.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgements
The Center for International Blood and Marrow Transplant Research is supported by grant U24-CA76518 from the National Cancer Institute, the National Heart, Lung, and Blood Institute, and the National Institute of Allergy and Infectious Diseases, National Institutes of Health, and by the Health Services Research Administration, Department of Health and Human Services (HHSH 250201200016C).
The content is solely the responsibility of the authors and does not represent the official policy of the National Institutes of Health or the Health Resources and Services Administration or any other agency of the US government.
Authorship
Contribution: R.B. and M.E. designed the study; R.B. performed the statistical analysis; and R.B., G.M.S., H.-L.W., B.C., A.R., C.D.F., J.S.H., J.K., J.J.M., J.A.P., D.R., S.S., M.C.W., J.E.W., J.F.T., E.G., and M.E. helped write the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Ruta Brazauskas, Division of Biostatistics, Institute for Health and Equity, Medical College of Wisconsin, 8701 Watertown Plank Rd, P.O. Box 26509, Milwaukee, WI 53226; e-mail: ruta@mcw.edu.
REFERENCES
Author notes
E.G. and M.E. are joint senior authors for this study.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal