Key Points
The induction of a profound antitumor response by T cells recognizing TAAs presented in self-HLA is possible, although exceptional.
High tetramer staining and peptide specificity are not solely predictive for relevant functional avidity of self-antigen–specific T cells.
Abstract
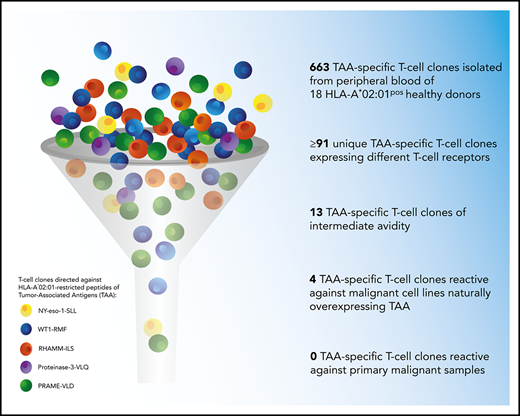
Tumor-associated antigens (TAAs) are monomorphic self-antigens that are proposed as targets for immunotherapeutic approaches to treat malignancies. We investigated whether T cells with sufficient avidity to recognize naturally overexpressed self-antigens in the context of self-HLA can be found in the T-cell repertoire of healthy donors. Minor histocompatibility antigen (MiHA)-specific T cells were used as a model, as the influence of thymic selection on the T-cell repertoire directed against MiHA can be studied in both self (MiHApos donors) and non-self (MiHAneg donors) backgrounds. T-cell clones directed against the HLA*02:01-restricted MiHA HA-1H were isolated from HA-1Hneg/HLA-A*02:01pos and HA-1Hpos/HLA-A*02:01pos donors. Of the 16 unique HA-1H–specific T-cell clones, five T-cell clones derived from HA-1Hneg/HLA-A*02:01pos donors and one T-cell clone derived from an HA-1Hpos/HLA-A*02:01pos donor showed reactivity against HA-1Hpos target cells. In addition, in total, 663 T-cell clones (containing at least 91 unique clones expressing different T-cell receptors) directed against HLA*02:01-restricted peptides of TAA WT1-RMF, RHAMM-ILS, proteinase-3-VLQ, PRAME-VLD, and NY-eso-1-SLL were isolated from HLA-A*02:01pos donors. Only 3 PRAME-VLD–specific and one NY-eso-1-SLL–specific T-cell clone provoked interferon-γ production and/or cytolysis upon stimulation with HLA-A*02:01pos malignant cell lines (but not primary malignant samples) naturally overexpressing the TAA. These results show that self-HLA–restricted T cells specific for self-antigens such as MiHA in MiHApos donors and TAAs are present in peripheral blood of healthy individuals. However, clinical efficacy would require highly effective in vivo priming by peptide vaccination in the presence of proper adjuvants or in vitro expansion of the low numbers of self-antigen–specific T cells of sufficient avidity to recognize endogenously processed antigen.
Introduction
Tumor-associated antigens (TAAs) are proposed as targets for immunotherapeutic approaches to treat malignancies.1-6 Most identified TAAs belong to the group of nonmutated monomorphic self-antigens such as differentiation antigens, aberrantly expressed antigens (eg, WT1, RHAMM, proteinase-3), and cancer-germline antigens (also known as cancer-testis antigens; eg, PRAME, NY-eso-1). Observations have suggested that T cells recognizing these self-antigens may contribute to antitumor reactivity after vaccination strategies or HLA-matched allogeneic hematopoietic stem cell transplantation (alloSCT). A relation has been proposed between expansion of TAA-specific T cells in the peripheral blood of patients after HLA-matched alloSCT and better relapse-free survival. Moreover, relapses have been observed in patients in the absence of TAA-specific T cells.7-12 These observations have generated interest in manipulating TAA-directed immunity to enhance antitumor reactivity.
Multiple phase 1/2 vaccination studies targeting TAAs in patients with hematologic malignancies have been performed.13-22 In a minority of vaccinated patients, clinical responses coincided with increased frequencies of TAA-specific T cells in peripheral blood. However, a causative relation between induction of high-avidity TAA-specific T cells and clinical effect has not been proven.23 In vitro studies have shown that T cells binding TAA-specific tetramers are regularly found in patients with hematologic malignancies and healthy donors.24-31 This observation suggests a role for TAA-specific T cells in the process of immune surveillance, whereby the immune system is supposed to identify and eliminate (pre)malignant cells.32,33 Functional analysis showed that TAA-specific T cells of both patients and donors were capable of recognizing target cells exogenously loaded with TAA peptides. However, it remains uncertain whether these T cells are capable of recognizing endogenously processed antigen.
Because TAA are self-antigens with low or restricted tissue-specific expression in nonmalignant cells, negative thymic selection is supposed to prevent autoimmunity by eliminating T cells with high avidity for self-antigens presented in self-HLA from the T-cell repertoire.34,35 However, dysregulated overexpression of TAAs in malignant cells may allow the immune system to discriminate these cells from their healthy counterparts, supporting the potential value of TAAs in immunotherapy.36-39 Minor histocompatibility antigens (MiHAs) are peptides derived from polymorphic genes that can be recognized as foreign by donor T cells when there is a disparity with the patient. After HLA-matched alloSCT, alloreactive T-cell responses directed against MiHA play a crucial role in the induction of a graft-versus-leukemia (GVL) effect and graft-versus-host disease.40-42 As expected, these functional MiHA-specific T-cell responses have exclusively been described in MiHApos patients receiving grafts from MiHAneg donors, because high-avidity MiHA-specific T cells are supposed to pass thymic selection only in MiHAneg donors.43-46 Because MiHA are considered self-antigens in MiHApos donors, it is expected that the MiHA-specific T-cell repertoire in MiHApos donors is shaped in a comparable way as the TAA-specific T-cell repertoire in healthy individuals.
To elucidate the antitumor potential of TAA-specific T cells, we investigated the self-HLA–restricted TAA-specific T-cell repertoire in healthy individuals. First, we assessed the influence of thymic selection on the shaping of the T-cell repertoire against self-antigens presented in self-HLA, using MiHA-specific T cells as a model. The polymorphic feature of MiHA allowed study of the T-cell repertoire directed against the same antigen in both a self (MiHApos donors) and non-self (MiHAneg donors) background, providing a proven positive control of high-avidity MiHA-specific T-cell responses. Because the hematopoiesis-restricted MiHA HA-1H has a balanced population frequency, this antigen was ideal for the current model.47 Second, we tested the functional reactivity of T cells recognizing TAAs presented in self-HLA and determined whether TAA-specific T cells that are able to recognize endogenously processed antigen are present in the T-cell repertoire of healthy individuals.
Materials and methods
Isolation of HA-1H–specific and TAA-specific T-cell clones
This study was conducted after approval was granted by the Leiden University Medical Center Institutional Board. After informed consent, peripheral blood mononuclear cells (PBMCs) were obtained to isolate TAA-specific T-cell clones recognizing 5 different TAAs presented in HLA-A*02:01 from 18 HLA-A*02:01pos donors and HA-1H–specific T-cell clones from 3 HA-1Hneg/HLA-A*02:01pos donors and 4 HA-1Hpos/HLA-A*02:01pos donors. The major histocompatibility complex class I (MHC-I)-Streptamer isolation technology as previously described (Table 1; supplemental Methods, available on the Blood Web site) was used.48 CD8pos/tetramerpos T cells underwent single-cell fluorescence-activated cell sorting and were expanded for functional analysis.
HLA-A*02:01-restricted TAAs and the MiHA included in this study
| Protein . | Peptide . | Peptide binding affinity for HLA-A*02:01, nM . |
|---|---|---|
| TAA | ||
| WT1 | RMFPNAPYL | 7.14 (SB) |
| RHAMM | ILSLELMKL | 160.52 (WB) |
| Proteinase-3 | VLQELNVTV | 12.33 (SB) |
| PRAME | VLDGLDVLL | 51.09 (WB) |
| NY-eso-1 | SLLMWITQV | 6.13 (WB) |
| MiHA | ||
| HA-1 | VLHDDLLEA | 28.61 (SB) |
| Protein . | Peptide . | Peptide binding affinity for HLA-A*02:01, nM . |
|---|---|---|
| TAA | ||
| WT1 | RMFPNAPYL | 7.14 (SB) |
| RHAMM | ILSLELMKL | 160.52 (WB) |
| Proteinase-3 | VLQELNVTV | 12.33 (SB) |
| PRAME | VLDGLDVLL | 51.09 (WB) |
| NY-eso-1 | SLLMWITQV | 6.13 (WB) |
| MiHA | ||
| HA-1 | VLHDDLLEA | 28.61 (SB) |
MHC I-Streptamers for isolation and tetramers for flow cytometry analysis were available for the indicated peptides. Peptide-binding affinities for HLA-A*02:01 were predicted by using NetMHC Version 4.0. SB are predicted strong binders, and WB are predicted weak binders.
Functional reactivity of T-cell clones
For stimulation assays, 2500 T cells were incubated with 15 000 stimulator cells (responder-to-stimulator [R:S] ratio, 1:6) in 384-well flat-bottom plates. After 18 hours, supernatants were harvested, and interferon-γ (IFN-γ) release was measured by using a standard enzyme-linked immunosorbent assay (Sanquin, Amsterdam, The Netherlands; and R&D Systems, Minneapolis, MN). For peptide titrations, stimulator cells were incubated with different concentrations of peptide for 1 hour at 37°C before the addition of T cells.
T-cell–mediated cytotoxicity was determined by using standard 51chromium release assays. Target cells were labeled with 100 μCi 51chromium (PerkinElmer, Waltham, MA) and, if indicated, loaded with peptide for 1 hour at 37°C, washed, and counted. A total of 2500 51chromium-labeled target cells were incubated with T cells at different effector-to-target (E:T) ratios in 96-well culture plates. After 4 hours of incubation, supernatants were harvested, transferred to Luma plates (PerkinElmer), and 51chromium release (cpm) was measured on a 2450 Microbeta2 plate counter (PerkinElmer). Maximal release was induced by 1% Triton (MilliporeSigma, Burlington, MA). The percentages of specific lysis were calculated with the following formula: [experimental release – spontaneous release]/[maximum release – spontaneous release)] × 100%.
Three previously identified CD8pos HA-1H–specific T-cell clones that were ex vivo selected from peripheral blood of patients with a profound GVL response after alloSCT and donor lymphocyte infusion (patient HA-1Hpos/HLA-A*0201pos and donor HA-1Hneg/HLA-A*0201pos) were included as high-avidity positive control in the indicated experiments (supplemental Methods).49 As positive control for HLA-A*02:01 expression and stimulatory capacity of malignant cell lines and primary malignant cells, an allo-HLA-A*02:01–reactive CD8pos T-cell clone was included in all experiments. The generation and culture of stimulator/target cells are described in the supplemental Methods. To induce artificial endogenous overexpression of TAAs in cell lines, 2 Epstein-Barr virus–transformed lymphoblastoid cell lines (EBV-LCL) were retrovirally transduced by using vectors encoding the full protein sequence of the respective TAA. TAA expression in cell lines and primary samples was analyzed by using quantitative reverse transcription polymerase chain reaction (RT-qPCR).
TRBV gene analysis and CDR3 sequencing
The T-cell receptor β variable (TRBV) gene usage of HA-1H–specific and TAA-specific T-cell clones was determined by using the IOTest Beta Mark Kit for TRBV repertoire analysis by flow cytometry (Immunotech SAS, a Beckman Coulter Company, Marseille, France) and indicated following the IMGT nomenclature.50 Because this kit has a coverage of 70% of the normal human TRBV genes, T-cell clones of which the TRBV gene could not be determined by this strategy were categorized as “TRBV?”. T-cell clones with the same antigen specificity, derived from the same donor, using the same TRBV gene and performing equally in the functional screenings, were considered identical T-cell clones. In cases in which the result of the functional screening varied in a group of T-cell clones, sequencing of the CDR3 region of the T-cell receptor β chain was performed on selected T-cell clones using anchoring reverse transcription of immunoreceptor sequences and amplification by nested polymerase chain reaction adapted for T-cell receptors (TCRs) (supplemental Methods).51-53
Results
Comparison of T-cell clones specific for HA-1H isolated from HA-1Hneg/HLA-A*02:01pos and HA-1Hpos/HLA-A*02:01pos donors
To investigate the effect of thymic selection on shaping of the T-cell repertoire directed against a self-antigen presented in self-HLA, MiHA-specific T-cell clones were used as a model. Because MiHAs are derived from polymorphic genes, the genetic background of an individual determines whether the MiHA is considered a non-self (MiHAneg donor) or self-antigen (MiHApos donor). By investigating in parallel the functional reactivity of MiHA-specific T-cell clones isolated from MiHAneg vs MiHApos donors, the shaping of the immune repertoire by thymic selection can be investigated.
MiHA HA-1H–specific CD8pos T-cell populations were enriched from PBMCs of three HA-1Hneg/HLA-A*02:01pos and four HA-1Hpos/HLA-A*02:01pos donors using the MHC-I-Streptamer technology and cloned by single cell fluorescence-activated cell sorting and expanded (supplemental Figure 1). Only CD8pos T-cell clones that stained clearly with the HA-1H tetramer and not with an irrelevant tetramer were included in further analyses. The numbers of unique T-cell clones were estimated by analysis of TRBV gene usage and subsequent sequencing of the TRB-CDR3 region for a selection of T-cell clones. HA-1H–specific T-cell clones were functionally screened by measuring cytokine release after stimulation with TAP-deficient T2 cells exogenously loaded with a titration of HA-1H peptide. Based on this analysis, T-cell clones were classified as high avidity (recognition of ≤10−10 M peptide), intermediate avidity (recognition of 10−9 M or 10−8 M peptide), low avidity (recognition of 10−7 M to 10−5 M peptide), or not functional (no recognition of 10−5 M peptide). As positive controls for high-avidity T-cell clones, 3 previously identified high-avidity CD8pos HA-1H–specific T-cell clones derived from an in vivo GVL response were included.49
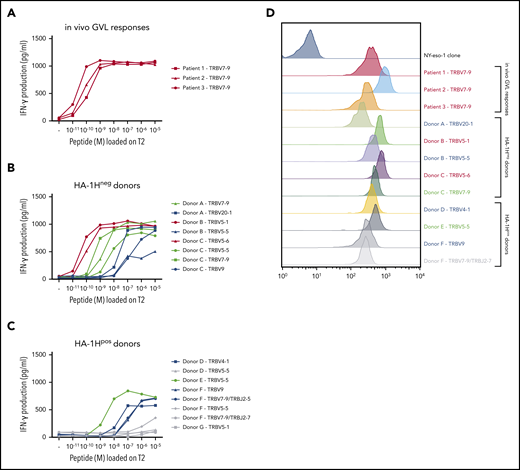
A total of 31 (≥8 unique) and 60 (≥8 unique) HA-1H–specific T-cell clones were generated from HA-1Hneg/HLA-A*02:01pos and HA-1Hpos/HLA-A*02:01pos donors, respectively (supplemental Table 1). The functional screening confirmed that the three T-cell clones isolated from in vivo GVL responses were indeed of high avidity, and all used the same TRBV7-9*01 gene (Figure 1A). Among the 8 HA-1H T-cell clones isolated from HA-1Hneg/HLA-A*02:01pos donors, 2 clones performed similar to the T-cell clones derived from in vivo GVL responses (Figure 1B, red curves) but had different TRB-CDR3 regions, with use of gene TRBV5-1*01 or TRBV5-6*01. The other six T-cell clones all required higher peptide concentrations for profound IFN-γ production: three T-cell clones were classified as intermediate-avidity clones (green curves), whereas the remaining 3 clones were of low avidity (blue curves). Among the HA-1H–specific T-cell clones derived from HA-1Hpos/HLA-A*02:01pos donors, only one T-cell clone was found to be of intermediate avidity (Figure 1C, green curve). The other seven T-cell clones exhibited low functional avidity (blue curves) or were not functional (gray curves). As illustrated in Figure 1D, only very subtle differences in intensities of tetramer staining were observed between high-, intermediate-, and low-avidity HA-1H–specific T-cell clones. High-avidity HA-1H–specific T-cell clones displayed overall a slightly higher tetramer staining compared with intermediate- and low-avidity HA-1H–specific T-cell clones. However, the intensity of tetramer staining of high-avidity HA-1H–specific T-cell clones isolated from patient 1 and patient 3 was comparable to several intermediate- and low-avidity HA-1H–specific T-cell clones.
Functional screening and tetramer staining of CD8pos HA-1H–specific T-cell clones. HA-1H–specific T-cell clones were stimulated with HLA-A*02:01pos TAP-deficient T2 cells exogenously loaded with a titration of the HA-1H peptide. IFN-γ production was assessed in a standard enzyme-linked immunosorbent assay (ELISA) (R:S ratio, 1:6). Clones in red were classified as high avidity, clones in green as intermediate avidity, clones in blue as low avidity, and clones in gray as not functional. Results of representative unique clones are depicted. (A) High avidity HA-1H–specific T-cell clones isolated from 3 patients during an in vivo GVL response after alloSCT. (B) HA-1H–specific T-cell clones isolated from 3 HA-1Hneg/HLA-A*02:01pos healthy donors. (C) HA-1H–specific T-cell clones isolated from 4 HA-1Hpos/HLA*02:01pos healthy donors. (D) Intensity of HA-1H tetramer staining for a selection of obtained HA-1H–specific T-cell clones. An NY-eso-1-SLL–specific T-cell clone was included as negative control.
Functional screening and tetramer staining of CD8pos HA-1H–specific T-cell clones. HA-1H–specific T-cell clones were stimulated with HLA-A*02:01pos TAP-deficient T2 cells exogenously loaded with a titration of the HA-1H peptide. IFN-γ production was assessed in a standard enzyme-linked immunosorbent assay (ELISA) (R:S ratio, 1:6). Clones in red were classified as high avidity, clones in green as intermediate avidity, clones in blue as low avidity, and clones in gray as not functional. Results of representative unique clones are depicted. (A) High avidity HA-1H–specific T-cell clones isolated from 3 patients during an in vivo GVL response after alloSCT. (B) HA-1H–specific T-cell clones isolated from 3 HA-1Hneg/HLA-A*02:01pos healthy donors. (C) HA-1H–specific T-cell clones isolated from 4 HA-1Hpos/HLA*02:01pos healthy donors. (D) Intensity of HA-1H tetramer staining for a selection of obtained HA-1H–specific T-cell clones. An NY-eso-1-SLL–specific T-cell clone was included as negative control.
These results show that HA-1H–specific T-cell clones showing proper staining with HA-1H tetramers and using a variety of TRBV genes can be isolated from both HA-1Hneg/HLA-A*02:01pos and HA-1Hpos/HLA-A*02:01pos donors. As expected, high-avidity HA-1H–specific T-cell clones were only isolated from HA-1Hneg/HLA-A*02:01pos donors, illustrating that the process of thymic selection has eliminated high-avidity T-cell clones in HA-1Hpos/HLA-A*02:01pos donors. However, T-cell clones of intermediate avidity are apparently allowed in the T-cell repertoire of both HA-1Hneg/HLA-A*02:01pos and HA-1Hpos/HLA-A*02:01pos donors. Furthermore, the intensity of tetramer staining was found to be not predictive for the functional reactivity of every HA-1H–specific T-cell clone.
Recognition of endogenously processed antigen by HA-1H–specific T-cell clones derived from HA-1Hneg/HLA-A*02:01pos and HA-1Hpos/HLA-A*02:01pos donors
We next investigated whether the functional classification of HA-1H–specific T-cell clones derived from HA-1Hneg/HLA-A*02:01pos and HA-1Hpos/HLA-A*02:01pos donors, based on their recognition of exogenously loaded HA-1H peptide, was indicative of their capacity to recognize endogenously processed and presented antigen. As positive control for HLA-A*02:01 expression and stimulatory capacity of the target cells, an allo-HLA-A*02:01–restricted T-cell clone was included in all functional tests.
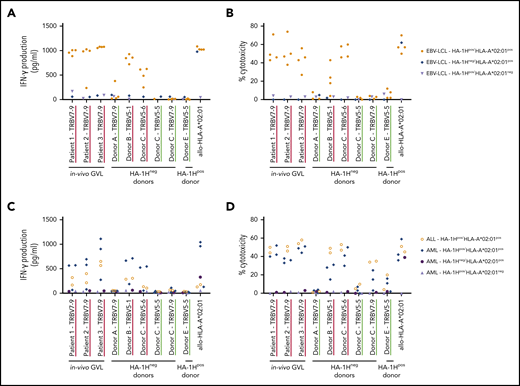
The HA-1H–specific T-cell clones that were classified as low avidity or not functional exhibited no reactivity against HA-1Hpos/HLA-A*02:01pos EBV-LCL (data not shown). As expected, the 3 high-avidity T-cell clones derived from in vivo GVL responses and the 2 high-avidity T-cell clones derived from HA-1Hneg/HLA-A*02:01pos donors clearly specifically recognized the HA-1Hpos/HLA-A*02:01pos EBV-LCL, illustrated by high concentrations of IFN-γ production (Figure 2A) and up to 70% of specific target cell lysis (Figure 2B). Of the 3 intermediate-avidity T-cell clones derived from HA-1Hneg donors, only T-cell clone “Donor A/TRBV7-9” showed marginal reactivity against one of the HA-1Hpos/HLA-A*02:01pos EBV-LCL, whereas the other two T-cell clones were not responsive. The intermediate-avidity T-cell clone derived from an HA-1Hpos donor did not exhibit IFN-γ production and only very marginal cytotoxic activity against HA-1Hpos/HLA-A*02:01pos EBV-LCL.
Recognition of endogenously processed HA-1H by high- and intermediate-avidity HA-1H–specific T-cell clones. T-cell clones underlined in red were classified as high avidity and clones underlined in green as intermediate avidity based on the functional screening with exogenously loaded peptide (Figure 1A-C). Results of representative unique clones are depicted. (A) IFN-γ production by HA-1H–specific T-cell clones after stimulation with an EBV-LCL panel containing 4 HA-1Hpos/HLA*02:01pos targets, 1 HA-1Hpos/HLA*02:01neg target, and 1 HA-1Hneg/HLA*02:01pos target (R:S ratio, 1:6). A representative example of 3 experiments is shown. (B) Cytotoxic activity of HA-1H–specific T-cell clones against the same EBV-LCL panel. Cytotoxicity was measured in a standard 51chromium-release assay at an E:T ratio of 10:1. Symbols represent median percentages of lysis of triplicates. (C) IFN-γ production by HA-1H–specific T-cell clones after stimulation with a panel of primary malignant samples, containing 2 HA-1Hpos /HLA*02:01pos ALL samples, 3 HA-1Hpos/HLA*02:01pos AML samples, 1 HA-1Hpos/HLA*02:01neg AML sample, and 1 HA-1Hneg/HLA*02:01pos AML sample (R:S ratio, 1:6). (D) Cytotoxic activity of HA-1H–specific T-cell clones against the same panel of primary malignant samples. Cytotoxicity was measured in a standard 51chromium-release assay (E:T ratio, 10:1). Symbols represent median percentages of lysis of triplicates.
Recognition of endogenously processed HA-1H by high- and intermediate-avidity HA-1H–specific T-cell clones. T-cell clones underlined in red were classified as high avidity and clones underlined in green as intermediate avidity based on the functional screening with exogenously loaded peptide (Figure 1A-C). Results of representative unique clones are depicted. (A) IFN-γ production by HA-1H–specific T-cell clones after stimulation with an EBV-LCL panel containing 4 HA-1Hpos/HLA*02:01pos targets, 1 HA-1Hpos/HLA*02:01neg target, and 1 HA-1Hneg/HLA*02:01pos target (R:S ratio, 1:6). A representative example of 3 experiments is shown. (B) Cytotoxic activity of HA-1H–specific T-cell clones against the same EBV-LCL panel. Cytotoxicity was measured in a standard 51chromium-release assay at an E:T ratio of 10:1. Symbols represent median percentages of lysis of triplicates. (C) IFN-γ production by HA-1H–specific T-cell clones after stimulation with a panel of primary malignant samples, containing 2 HA-1Hpos /HLA*02:01pos ALL samples, 3 HA-1Hpos/HLA*02:01pos AML samples, 1 HA-1Hpos/HLA*02:01neg AML sample, and 1 HA-1Hneg/HLA*02:01pos AML sample (R:S ratio, 1:6). (D) Cytotoxic activity of HA-1H–specific T-cell clones against the same panel of primary malignant samples. Cytotoxicity was measured in a standard 51chromium-release assay (E:T ratio, 10:1). Symbols represent median percentages of lysis of triplicates.
To investigate whether the high- and/or intermediate-avidity HA-1H–specific T-cell clones were able to recognize HA-1Hpos/HLA-A*02:01pos malignant cells, these T-cell clones were tested against primary acute myeloid leukemia (AML) and acute lymphoblastic leukemia (ALL) samples containing 65% to 100% blasts. Again, the 3 high-avidity T-cell clones derived from in vivo GVL responses and the 2 high-avidity T-cell clones derived from HA-1Hneg/HLA-A*02:01pos donors exhibited profound IFN-γ production (Figure 2C) and cytolytic activity (Figure 2D) against HA-1Hpos/HLA-A*02:01pos malignant cells. Of the intermediate-avidity T-cell clones derived from HA-1Hneg/HLA-A*02:01pos donors, T-cell clones “Donor C/TRBV7-9” and “Donor C/TRBV5-5” surprisingly exhibited minimal/moderate cytolytic activity against the HA-1Hpos/HLA-A*02:01pos primary malignant samples with no coinciding IFN-γ production, whereas these T-cell clones were not reactive against the HA-1Hpos/HLA-A*02:01pos EBV-LCL. A similar phenomenon was observed for T-cell clone “Donor E/TRBV5-5” derived from an HA-1Hpos/HLA-A*02:01pos donor.
These results show that the HA-1H–specific T-cell repertoire of HA-1Hneg/HLA-A*02:01pos donors contains high- and intermediate-avidity T-cell clones that are able to recognize endogenously processed and presented peptide. HA-1H–specific clones derived from HA-1Hpos/HLA-A*02:01pos donors classified as intermediate avidity showed only limited HA-1H–specific reactivity.
The functional reactivity of self-HLA-restricted TAA-specific T-cell clones resembled the functional reactivity of HA-1H–specific T-cell clones derived from HA-1Hpos/HLA-A*02:01pos donors
Because TAAs are self-antigens, the shaping of the TAA-specific T-cell repertoire in HLA-A*02:01pos healthy donors by thymic selection is expected to resemble the selection seen for the HA-1H–specific T-cell repertoire in HA-1Hpos/HLA-A*02:01pos donors. To investigate the repertoire of T cells recognizing TAAs in the context of self-HLA in healthy donors, CD8pos T-cell clones directed against the HLA-A*02:01–restricted peptides of WT1-RMF, RHAMM-ILS, proteinase-3-VLQ, PRAME-VLD, and NY-eso-1-SLL were isolated from PBMCs of HLA-A*02:01pos donors (Table 1). All CD8pos/tetramerpos T-cell clones were functionally screened for antigen-specific reactivity measured by cytokine release after stimulation with T2 cells exogenously loaded with a titration of the respective peptide; they were accordingly classified as high avidity, intermediate avidity, low avidity, or not functional. Their TRBV gene usage was assessed by using flow cytometry. T-cell clones with the same antigen specificity, derived from the same donor, using the same TRBV gene and performing equally in the functional screening, were categorized as identical T-cell clones. T-cell clones from the same donor using the same TRBV gene but displaying variable reactivity in the functional screenings were sequenced for their TRB-CDR3 region to identify different unique T-cell clones.
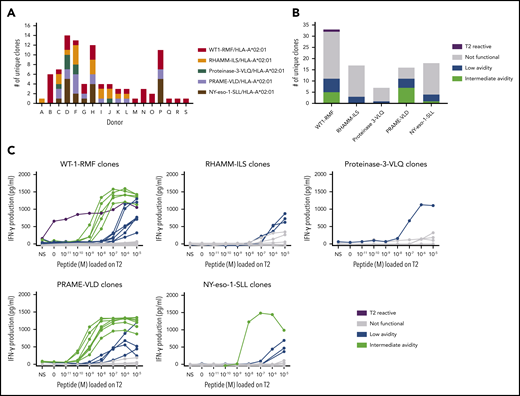
A total of 663 TAA-specific T-cell clones were isolated from 18 donors, among which were 301 WT1-RMF–specific T-cell clones from 17 donors, 129 RHAMM-ILS–specific T-cell clones from 9 donors, 36 proteinase-3-VLQ–specific T-cell clones from 4 donors, 114 PRAME-VLD–specific T-cell clones from 9 donors, and 83 NY-eso-1-SLL–specific T-cell clones from 6 donors. All CD8pos TAA-specific T-cell clones stained only with the relevant tetramers and not with irrelevant HLA-A*02:01-restricted tetramers (representative examples in supplemental Figure 2A). Within the 663 T-cell clones, a minimum of 91 unique T-cell clones were identified, including 33 WT1-RMF, 17 RHAMM-ILS, 7 proteinase-3-VLQ, 16 PRAME-VLD, and 18 NY-eso-1-SLL–specific T-cell clones (Figure 3A; supplemental Tables 2-6, respectively). The numbers of isolated unique TAA-specific T-cell clones per specificity did not correlate with the strength of the predicted binding affinity of the peptide to the HLA-A*02:01 molecule (Table 1). The functional screening revealed that no high-avidity T-cell clones were isolated from any of the 18 donors for any of the 5 TAA specificities. Sixty unique T-cell clones (66%) were shown to be not functional (Figure 3B), 17 unique T-cell clones (19%) were classified as low avidity, and 1 WT1-RMF clone (1%) displayed profound reactivity against T2 cells without loading of WT1-RMF peptide, indicating cross-reactivity. However, 13 unique T-cell clones (14%) were categorized as intermediate avidity, including 5 WT1-RMF, 7 PRAME-VLD, and 1 NY-eso-1-SLL T-cell clones (Figure 3C). These T-cell clones showed no reactivity against irrelevant HLA-A*02:01-restricted peptides (supplemental Figure 2B). The intensity of tetramer staining did not correlate with the functional avidity of the TAA-specific T-cell clones (data not shown).
Functional screening of CD8pos TAA-specific T-cell clones derived from HLA-A*02:01pos donors. (A) Minimal numbers of unique T-cell clones per TAA specificity isolated from 18 donors. (B) Summary of classification of unique TAA-specific T-cell clones based on IFN-γ production after overnight stimulation with TAP-deficient T2 cells exogenously loaded with a titration of the respective peptide (R:S ratio, 1:6). (C) Results of this functional screening per TAA specificity. Clones depicted in green were classified as intermediate avidity, clones depicted in blue as low avidity, clones depicted in gray as not functional, and clones depicted in purple as T2-reactive T-cell clones. Representative examples of unique clones are depicted. NS, not stimulated.
Functional screening of CD8pos TAA-specific T-cell clones derived from HLA-A*02:01pos donors. (A) Minimal numbers of unique T-cell clones per TAA specificity isolated from 18 donors. (B) Summary of classification of unique TAA-specific T-cell clones based on IFN-γ production after overnight stimulation with TAP-deficient T2 cells exogenously loaded with a titration of the respective peptide (R:S ratio, 1:6). (C) Results of this functional screening per TAA specificity. Clones depicted in green were classified as intermediate avidity, clones depicted in blue as low avidity, clones depicted in gray as not functional, and clones depicted in purple as T2-reactive T-cell clones. Representative examples of unique clones are depicted. NS, not stimulated.
These results show that the majority of self-HLA–restricted TAA-specific T-cell clones in the repertoire of healthy donors were not functional or of low avidity and that only 13 of 91 unique T-cell clones were of intermediate avidity. These findings are in line with our observations for HA-1H–specific T-cell clones derived from HA-1Hpos/HLA-A*02:01pos donors.
A minority of intermediate-avidity TAA-specific T-cell clones is able to recognize cell lines naturally overexpressing TAA
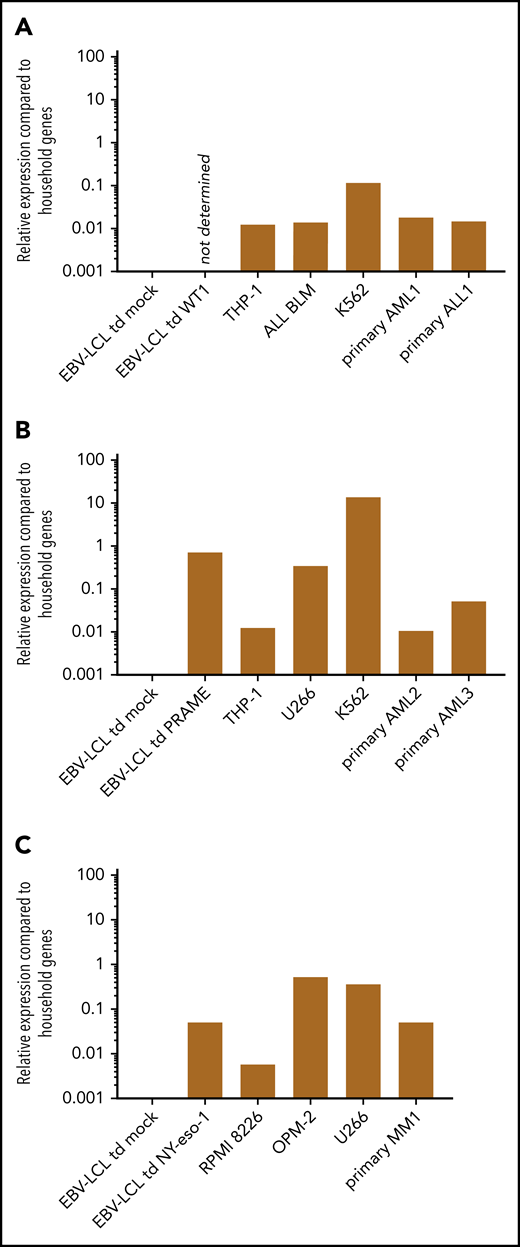
In contrast to MiHAs, TAAs are expected to be overexpressed in malignant cells compared with healthy cells. This TAA overexpression might influence the threshold for TAA-specific T-cell activation and recognition of endogenously processed antigen. We therefore investigated whether WT1-RMF, PRAME-VLD, and NY-eso-1-SLL–specific T-cell clones of intermediate avidity were able to recognize endogenously processed antigen. Two HLA-A*02:01pos EBV-LCL were transduced with a retroviral vector encoding the full protein sequence of WT1, PRAME, or NY-eso-1 to induce overexpression of the respective TAAs. Furthermore, tumor cell lines known for their natural TAA overexpression and several primary ALL and AML samples were included. Relative TAA expression in these targets compared with household genes was quantified by using RT-qPCR. Both cytokine production and cytotoxic capacity of T-cell clones were assessed.
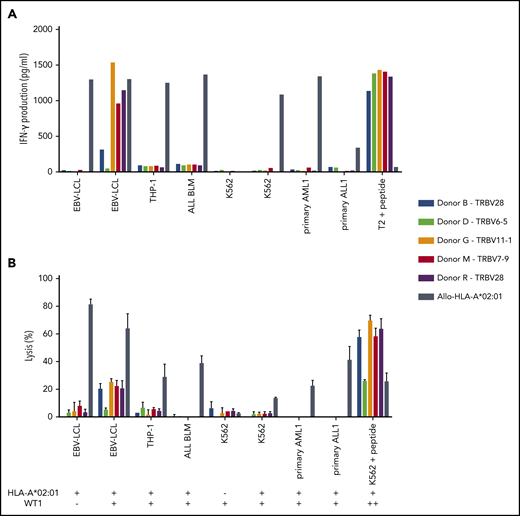
Of the 5 intermediate-avidity WT1-RMF–specific T-cell clones, three T-cell clones (“Donor G/TRBV11-1,” “Donor M/TRBV7-9,” and “Donor R/TRBV28”) produced high levels of IFN-γ and one T-cell clone (“Donor B/TRBV28”) produced low levels of IFN-γ upon stimulation with EBV-LCL transduced with WT1 and not EBV-LCL transduced with the mock vector; stimulation with peptide-loaded stimulator cells, however, provoked profound IFN-γ production (Figure 4A). Different malignant cell lines and primary malignant samples with limited relative WT1 expression did not provoke significant IFN-γ production (Figure 5A). A similar reactivity pattern was seen when the cytotoxic activity of WT1-specific T-cell clones was assessed, showing limited lysis of EBV-LCL transduced with WT1 and profound lysis of peptide-loaded target cells (Figure 4B).
Recognition of endogenously processed WT1 by intermediate-avidity WT1-RMF–specific T-cell clones. The 5 WT1-RMF–specific T-cell clones of intermediate avidity were tested against EBV-LCL transduced with the empty vector (mock) or with the vector encoding the full protein sequence of WT1, AML cell line THP-1, ALL cell line BLM, and chronic myeloid leukemia cell line K562 wild-type and transduced with HLA-A*02:01, 5 primary AML samples, and 3 primary ALL samples (2 representative primary samples with relatively high WT1 expression are depicted). An allo-HLA-A*02:01–reactive T-cell clone was included as positive control for HLA-A*02:01 expression and costimulatory capacity of the target cells. T2 or K562 transduced with HLA-A*02:01 and loaded with peptide were included as positive control for functional reactivity of clones. The HLA-A*02:01 and TAA expression of the target cells is indicated as negative (–) or positive (+) below the x-axis. (A) IFN-γ production was measured by using an ELISA after overnight stimulation (R:S ratio, 1:6). Representative example of 3 experiments is shown. (B) Cytotoxic capacity was measured in a standard 51chromium-release assay (E:T ratio, 10:1). Mean of triplicates with standard deviation is depicted.
Recognition of endogenously processed WT1 by intermediate-avidity WT1-RMF–specific T-cell clones. The 5 WT1-RMF–specific T-cell clones of intermediate avidity were tested against EBV-LCL transduced with the empty vector (mock) or with the vector encoding the full protein sequence of WT1, AML cell line THP-1, ALL cell line BLM, and chronic myeloid leukemia cell line K562 wild-type and transduced with HLA-A*02:01, 5 primary AML samples, and 3 primary ALL samples (2 representative primary samples with relatively high WT1 expression are depicted). An allo-HLA-A*02:01–reactive T-cell clone was included as positive control for HLA-A*02:01 expression and costimulatory capacity of the target cells. T2 or K562 transduced with HLA-A*02:01 and loaded with peptide were included as positive control for functional reactivity of clones. The HLA-A*02:01 and TAA expression of the target cells is indicated as negative (–) or positive (+) below the x-axis. (A) IFN-γ production was measured by using an ELISA after overnight stimulation (R:S ratio, 1:6). Representative example of 3 experiments is shown. (B) Cytotoxic capacity was measured in a standard 51chromium-release assay (E:T ratio, 10:1). Mean of triplicates with standard deviation is depicted.
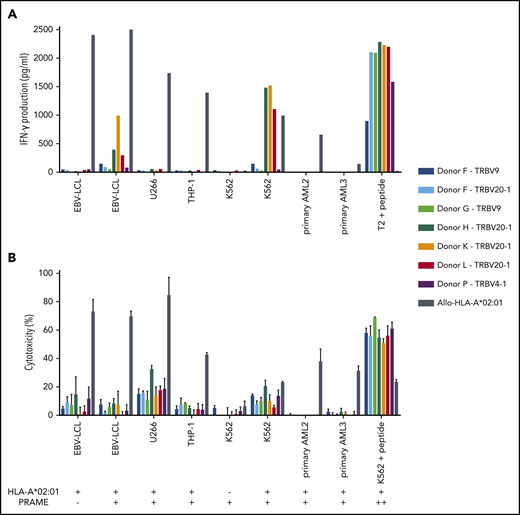
Three of the 7 PRAME-VLD–specific T-cell clones of intermediate avidity (“Donor H/TRBV20-1,” “Donor K/TRBV20-1,” and “Donor L/TRBV20-1”) exhibited moderate IFN-γ production after stimulation with the EBV-LCL transduced with PRAME and high IFN-γ production upon stimulation with the PRAME-overexpressing malignant cell line K562 or peptide-loaded stimulator cells (Figures 5B and 6A). PRAME-VLD–specific T-cell clones showed only limited cytotoxic activity against the cell line targets, whereas none of the tested primary AML and ALL samples were recognized (Figure 6B), despite moderate PRAME expression. Peptide-loaded target cells were properly killed.
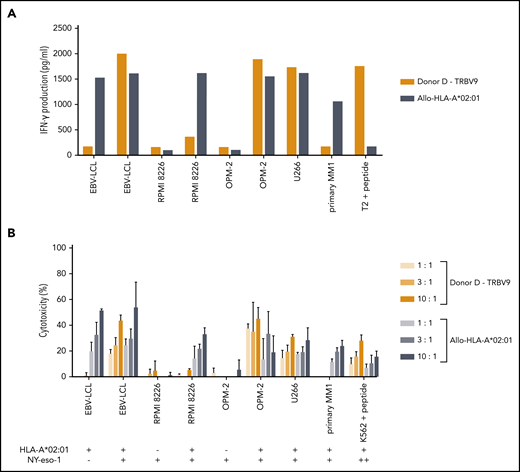
The single intermediate-avidity NY-eso-1-SLL–specific T-cell clone “Donor D/TRBV9” clearly recognized EBV-LCL transduced with NY-eso-1 and multiple myeloma (MM) cell lines OPM-2 and U266 transduced HLA-A*02:01 that endogenously overexpressed NY-eso-1 (Figures 5C and 7A), but not the primary MM sample, which showed NY-eso-1 expression comparable to the EBV-LCL transduced with NY-eso-1. Cytolytic capacity of the NY-eso-1-SLL–specific T-cell clone was illustrated by profound lysis of NY-eso-1pos/HLA-A*02:01pos cell lines to an extent comparable to that of the allo-HLA-A*02:01–specific T-cell clone, even at low effector-to-target ratios (Figure 7B). However, no lysis of the primary MM sample could be shown despite proper recognition by the allo-HLA-A*02:01–specific T-cell clone.
Relative expression of TAA in different target cell lines and primary samples measured by RT-qPCR. The expression of the indicated TAA is calculated relative to expression of 2 household genes (ACTB and GAPDH). A relative expression of 1 means equal expression compared with the average expression of these household genes. (A) The expression of WT1 could not be determined in EBV-LCL transduced with the retroviral vector encoding the codon-optimized protein sequence of WT1, as a suitable primer was not available. Expression of WT1 in EBV-LCL transduced mock, THP-1, ALL BLM, and K562 cell line and 2 representative primary AML and ALL samples are depicted. (B) Expression of PRAME in EBV-LCL transduced mock and PRAME, THP-1, U266, K562 cell line, and 2 representative primary AML samples are depicted. (C) Expression of NY-eso-1 in EBV-LCL transduced mock and NY-eso-1, MM cell lines RPMI 8226, OPM-2, and U266, and 1 representative primary MM sample are depicted.
Relative expression of TAA in different target cell lines and primary samples measured by RT-qPCR. The expression of the indicated TAA is calculated relative to expression of 2 household genes (ACTB and GAPDH). A relative expression of 1 means equal expression compared with the average expression of these household genes. (A) The expression of WT1 could not be determined in EBV-LCL transduced with the retroviral vector encoding the codon-optimized protein sequence of WT1, as a suitable primer was not available. Expression of WT1 in EBV-LCL transduced mock, THP-1, ALL BLM, and K562 cell line and 2 representative primary AML and ALL samples are depicted. (B) Expression of PRAME in EBV-LCL transduced mock and PRAME, THP-1, U266, K562 cell line, and 2 representative primary AML samples are depicted. (C) Expression of NY-eso-1 in EBV-LCL transduced mock and NY-eso-1, MM cell lines RPMI 8226, OPM-2, and U266, and 1 representative primary MM sample are depicted.
Functional reactivity of intermediate-avidity PRAME-VLD–specific T-cell clones against targets endogenously overexpressing PRAME. The 7 PRAME-VLD–specific T-cell clones of intermediate avidity were tested against EBV-LCL transduced with the empty vector (mock) or with the vector encoding the full protein sequence of PRAME, MM cell line U266, AML cell line THP-1, and the chronic myeloid leukemia cell line K562 wild-type or transduced with HLA-A*02:01, 6 primary AML samples, and 2 primary ALL samples (2 representative primary samples with relatively high WT1 expression are shown). An allo-HLA-A*02:01–reactive T-cell clone was included as positive control for HLA-A*02:01 expression and costimulatory capacity of the target cells. T2 or K562 transduced with HLA-A*02:01 and loaded with peptide were included as positive control for functional reactivity of clones. The HLA-A*02:01 and TAA expression of the targets is indicated as negative (–) or positive (+) below the x-axis. (A) IFN-γ production was measured by using an ELISA after overnight stimulation (R:S ratio, 1:6). Representative example of 3 experiments is shown. (B) Cytotoxic capacity was measured in a standard 51chromium-release assay (E:T ratio, 10:1). Mean of triplicates with standard deviation is depicted.
Functional reactivity of intermediate-avidity PRAME-VLD–specific T-cell clones against targets endogenously overexpressing PRAME. The 7 PRAME-VLD–specific T-cell clones of intermediate avidity were tested against EBV-LCL transduced with the empty vector (mock) or with the vector encoding the full protein sequence of PRAME, MM cell line U266, AML cell line THP-1, and the chronic myeloid leukemia cell line K562 wild-type or transduced with HLA-A*02:01, 6 primary AML samples, and 2 primary ALL samples (2 representative primary samples with relatively high WT1 expression are shown). An allo-HLA-A*02:01–reactive T-cell clone was included as positive control for HLA-A*02:01 expression and costimulatory capacity of the target cells. T2 or K562 transduced with HLA-A*02:01 and loaded with peptide were included as positive control for functional reactivity of clones. The HLA-A*02:01 and TAA expression of the targets is indicated as negative (–) or positive (+) below the x-axis. (A) IFN-γ production was measured by using an ELISA after overnight stimulation (R:S ratio, 1:6). Representative example of 3 experiments is shown. (B) Cytotoxic capacity was measured in a standard 51chromium-release assay (E:T ratio, 10:1). Mean of triplicates with standard deviation is depicted.
Functional reactivity of the NY-eso-1-SLL–specific T-cell clone of intermediate avidity against cell lines endogenously overexpressing NY-eso-1. NY-eso-1-SLL clone “Donor D-TRBV9” was tested against EBV-LCL transduced with the empty vector (mock) or with the vector encoding the full protein sequence of NY-eso-1, MM cell lines RPMI 8226 transduced with the empty vector (mock) or with HLA-A*02:01, MM cell line OPM-2 transduced with the empty vector (mock) or with HLA-A*02:01, MM cell line U266, and a primary MM sample. An allo-HLA-A*02:01–reactive T-cell clone was included as positive control for HLA-A*02:01 expression and costimulatory capacity of the targets. T2 or K562 transduced with HLA-A*02:01 and loaded with peptide were included as positive control. The HLA-A*02:01 and TAA expression of the targets is indicated as negative (–) or positive (+) below the x-axis. (A) IFN-γ production was measured by using an ELISA after overnight stimulation (R:S ratio, 1:6). Representative example of 3 experiments is shown. (B) Cytotoxic capacity was measured in a standard 51chromium-release assay at different E:T ratios. Mean of triplicates with standard deviation is depicted.
Functional reactivity of the NY-eso-1-SLL–specific T-cell clone of intermediate avidity against cell lines endogenously overexpressing NY-eso-1. NY-eso-1-SLL clone “Donor D-TRBV9” was tested against EBV-LCL transduced with the empty vector (mock) or with the vector encoding the full protein sequence of NY-eso-1, MM cell lines RPMI 8226 transduced with the empty vector (mock) or with HLA-A*02:01, MM cell line OPM-2 transduced with the empty vector (mock) or with HLA-A*02:01, MM cell line U266, and a primary MM sample. An allo-HLA-A*02:01–reactive T-cell clone was included as positive control for HLA-A*02:01 expression and costimulatory capacity of the targets. T2 or K562 transduced with HLA-A*02:01 and loaded with peptide were included as positive control. The HLA-A*02:01 and TAA expression of the targets is indicated as negative (–) or positive (+) below the x-axis. (A) IFN-γ production was measured by using an ELISA after overnight stimulation (R:S ratio, 1:6). Representative example of 3 experiments is shown. (B) Cytotoxic capacity was measured in a standard 51chromium-release assay at different E:T ratios. Mean of triplicates with standard deviation is depicted.
These results show that of the 13 intermediate-avidity TAA-specific T-cell clones, 3 PRAME-VLD–specific T-cell clones and 1 NY-eso-1-SLL–specific T-cell clone exhibited IFN-γ production upon stimulation with malignant cell lines naturally overexpressing the corresponding TAAs. Only for the NY-eso-1-SLL–specific T-cell clone was clear cytotoxic activity against malignant cell lines shown. However, primary malignant samples displaying proper TAA gene expression were not recognized by any of the intermediate-avidity TAA-specific T-cell clones.
Discussion
The current study investigated whether significant antitumor responses by self-HLA–restricted TAA-specific T cells isolated from the autologous or HLA-matched T-cell repertoire are likely to be induced in the setting of immunotherapeutic approaches. We described the role of thymic selection on shaping of the T-cell repertoire directed against self-antigens presented in self-HLA using HA-1H–specific T-cell clones isolated from HA-1Hneg/HLA-A*02:01pos vs HA-1Hpos/HLA-A*02:01pos donors as a model. Furthermore, HLA-A*02:01–restricted TAA-specific T cells were isolated from the T-cell repertoire of HLA-A*02:01pos healthy donors, and it was shown that only a small minority of isolated TAA-specific T-cell clones were able to recognize malignant cell lines expressing endogenously processed TAA but not primary malignant samples. These results illustrate that the induction of a profound antitumor response by T cells recognizing TAA presented in self-HLA is possible, although very exceptional.
We compared the functionality of MiHA HA-1H–specific T-cell clones isolated from HA-1Hneg/HLA-A*02:01pos vs HA-1Hpos/HLA-A*02:01pos donors. Overall, the capacity of HA-1H–specific T-cell clones to recognize stimulator cells exogenously loaded with low concentrations of the peptide appeared indicative for their reactivity against stimulator cells presenting endogenously processed antigen. As expected, high-avidity HA-1H–specific T-cell clones that recognized HA-1Hpos/HLA-A*02:01pos primary malignant samples to the same extent as HA-1H–specific T-cell clones derived from an in vivo immune response were only obtained from HA-1Hneg/HLA-A*02:01pos donors and not from HA-1Hpos/HLA-A*02:01pos donors. However, one HA-1H–specific T-cell clone isolated from HA-1Hpos/HLA-A*02:01pos donors showed limited cytolytic activity against HA-1Hpos/HLA-A*02:01pos targets, indicating that self-HLA–restricted T-cell clones with an intermediate avidity that are able to recognize endogenously processed self-antigens can pass thymic selection and become part of the T-cell repertoire. Interestingly, TCR sequencing revealed that the TCRs of the two high-avidity HA-1H–specific T-cell clones isolated from HA-1Hneg/HLA-A*02:01pos donors used the TRBV5-1*01 and TRBV5-6*01 gene, respectively. Previous studies reported that both ex vivo generated and in vivo selected HA-1H–specific T-cells preferentially use the TRBV7-9 gene for their TCR configuration, in combination with a variety of TRBJ genes.44,54-58
The similar model was used to test 663 T-cell clones (containing at least 91 unique T-cell clones expressing different TCRs) directed against HLA*A*02:01–restricted TAAs WT1-RMF, RHAMM-ILS, proteinase-3-VLQ, PRAME-VLD, and NY-eso-1-SLL isolated from several HLA-A*02:01pos healthy donors. In agreement with our findings regarding HA-1H–specific T-cell clones derived from HA-1Hpos/HLA-A*02:01pos donors, we found no self-HLA–restricted TAA-specific T-cell clones classified as high avidity based on recognition of stimulator cells exogenously loaded with the relevant peptide. This observation confirmed the role of negative thymic selection for TAA-specific T cells restricted to self-HLA. We identified many different low-avidity or nonfunctional HA-1H–specific and TAA-specific T-cell clones that clearly stained with the corresponding tetramer. In line with previous research, the intensity of tetramer staining did not completely correlate with functional reactivity.29 These observations might explain the discrepancy between the in vivo appearance of tetramerpos TAA-specific T cells seen in TAA peptide vaccination studies and the lack of convincing coinciding antitumor reactivity in the majority of studied patients.16,23,59 Our findings show that identifying tetramer-positive TAA-specific T-cell responses in vivo does not prove antitumor effect but may be the result of expansion of clonotypes with relatively low functional avidity without the ability to induce clinically relevant immune reactions.
The extensive pool of isolated TAA-specific T-cell clones contained only a limited number of intermediate-avidity T-cell clones. These T-cell clones were specific for WT1-RMF, PRAME-VLD, or NY-eso-1-SLL, whereas for proteinase-3-VLQ and RHAMM-ILS only low-avidity T-cell clones were identified in this study. An explanation for this discrepancy can be found in the overall lower numbers of isolated proteinase-3-VLQ– and RHAMM-ILS–specific T-cell clones compared with WT-1-RMF–, PRAME-VLD–, and NY-eso-1-SLL–specific T-cell clones, reducing the chance of catching an intermediate-avidity T-cell clone. The numbers of isolated T-cell clones per TAA specificity did not correlate with the predicted binding affinities of the TAA peptides for HLA-A*02:01, as WT1-RMF and proteinase-3-VLQ are predicted strong binders, whereas RHAMM-ILS, PRAME-VLD, and NY-eso-1-SLL are predicted weak binders. Therefore, the capacity of the specific TAA-peptides to compete for presentation in HLA-A*02:01 was not predictive for the numbers of isolated T-cell clones for the different specificities. Differences in expression levels between TAA in medullary thymic epithelial cells can affect the threshold for negative thymic selection of TAA-specific T cells, thereby influencing the avidity of T cells present in the T-cell repertoire.60 For TAA-specific T cells that pass thymic selection, we hypothesized that TAA-overexpressing targets can positively influence their activation threshold, resulting in activation of not only high-avidity but also intermediate-avidity T cells, while preserving tolerance against healthy cells. Although all 13 intermediate-avidity T-cell clones were comparable in the functional tests against peptide-loaded target cells, only 3 PRAME-VLD– and 1 NY-eso-1-SLL–specific T-cell clone exhibited reactivity against malignant cell lines. An explanation may be found in the extent of relative TAA overexpression in the tested malignant cell lines. The TAA expression on the RNA level of PRAME in K562 and NY-eso-1 in U266 was found to be higher than or comparable to the expression of the household genes.10,28,61 In contrast, the relative expression of WT1 in K562 was only 0.12-fold the expression of the household genes and even lower in the tested primary malignant samples, as also described previously.36 In line with this, the intermediate-avidity PRAME-VLD–specific T-cell clones were not able to recognize primary malignant leukemic samples that exhibited much lower RNA expression levels of PRAME than K562 cells. However, the NY-eso-1-SLL T-cell clone of intermediate avidity was not able to recognize the tested MM sample, although the NY-eso-1 expression on the RNA level of these primary malignant cells was comparable to the expression in the EBV-LCL transduced with NY-eso-1, which were clearly recognized. These observations suggest that in addition to relatively high TAA expression on the RNA level of malignant cells, additional factors (eg, processing and peptide presentation) most likely dictate proper recognition of primary malignant cells. The gene expression on the RNA level might not always correlate to the actual protein expression on the cell surface, as intracellular translation and protein degradation are not taken into account.62 In addition, because malignant cells have been shown to be a heterogeneous population of cells with distinct gene expression profiles, TAA expression on the RNA level might differ among malignant cells.61,63 This might result in recognition or killing of only a selection of malignant cells with high TAA expression.
Clinically relevant immune responses targeting cancer-germline antigens such as PRAME and NY-eso-1 have been reported in patients with cancer-germline–expressing hematologic cancers.64 Our data show that the induction of functional TAA-specific T-cell responses from the unmanipulated autologous or HLA-matched T-cell repertoire might be possible but is difficult due to the low frequencies of TAA-specific T-cells that have the potential to recognize naturally overexpressed antigen in malignant cells. Therefore, a very profound immune response has to be induced to enhance the chance of a functionally significant and clinically relevant TAA-specific T-cell response. The addition of highly effective adjuvants to peptide vaccination strategies targeting TAA may increase the efficiency of priming of TAA-specific T cells of sufficient functional avidity to recognize endogenously processed antigens presented on malignant cells. In vitro isolation and expansion of high-avidity TAA-specific T cells may be another strategy to use the potential of these TAA-specific T cells that seem to be present only at very low frequencies. Although these approaches have been shown to be safe and feasible in phase 1/2 studies, convincing evidence regarding the induction of high-avidity epitope-specific T-cell responses after TAA-specific peptide vaccination or adoptive transfer of TAA-specific T-cells in patients with hematologic malignancies has not been reported thus far; strategies may require further improvement, however.23,59,65-68 Multiple recently initiated trials focusing on TAAs are expected to provide additional insight regarding the potential of autologous TAA-specific T cells for the induction of clinically relevant antitumor responses in patients with hematologic malignancies.69-73
In conclusion, we have shown that self-HLA–restricted TAA-specific T cells can be easily isolated from peripheral blood of healthy individuals and that only exceptional T-cell clones are capable of recognizing naturally overexpressed antigen in malignant cell lines but not primary malignant cells. Therefore, classification of functional TAA-specific T cells by only high tetramer staining and peptide specificity leads to the overestimation of relevant avidity of these T cells.
Sequencing data are available on the European Nucleotide Archive at EMBL-EBI (accession number PRJEB38225).
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank the employees of the Laboratory for Specialized Hematology and the Flowcytometry Core Facility, Kees van Bergen and Renate Hagedoorn (Leiden University Medical Center), for providing expert technical assistance.
This study was financially supported by the European Union’s Seventh Framework Programme (FP/2007-2013; grant 601722) and by the Dutch Cancer Society (grant UL 2008-4263).
Authorship
Contribution: M.C.J.R. designed, performed, analyzed, and interpreted all experiments and wrote the manuscript; L.H. performed and interpreted experiments, constructed retroviral expression vectors, performed quantitative reverse transcription polymerase chain reaction, and sequenced TCRs; S.A.J.V., E.v.E., and C.H. performed experiments; L.G. and C.S. provided the MHC-I-Streptamer technology equipment; H.E. designed the study; J.H.F.F. designed and supervised the study, and wrote the manuscript; and I.J. designed and supervised the study, analyzed and interpreted experiments, and wrote the manuscript. All authors reviewed the manuscript.
Conflict-of-interest disclosure: L.G. is senior vice president and managing director of Juno Therapeutics GmbH. C.S. is an employee of Juno Therapeutics GmbH. The remaining authors declare no competing financial interests.
Correspondence: Marthe C. J. Roex, Department of Hematology, Leiden University Medical Center, C2R-140, PO Box 9600, 2300 RC Leiden, The Netherlands; e-mail: m.c.j.roex@lumc.nl.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal