Key Points
Transient use of Tpo-RAs for refractory ITP in pregnancy seems relatively safe and effective for mother and fetus.
Abstract
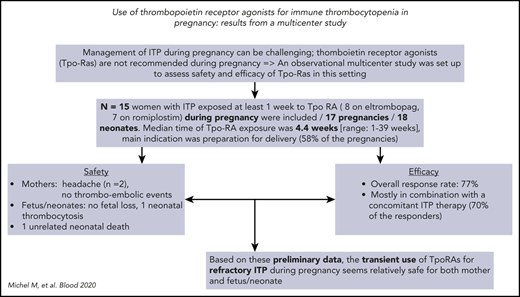
Management of immune thrombocytopenia (ITP) during pregnancy can be challenging because treatment choices are limited. Thrombopoietin receptor agonists (Tpo-RAs), which likely cross the placenta, are not recommended during pregnancy. To better assess the safety and efficacy of off-label use of Tpo-RAs during pregnancy, a multicenter observational and retrospective study was conducted. Results from 15 pregnant women with ITP (pregnancies, n = 17; neonates, n = 18) treated with either eltrombopag (n = 8) or romiplostim (n = 7) during pregnancy, including 2 patients with secondary ITP, were analyzed. Median time of Tpo-RA exposure during pregnancy was 4.4 weeks (range, 1-39 weeks); the indication for starting Tpo-RAs was preparation for delivery in 10 (58%) of 17 pregnancies, whereas 4 had chronic refractory symptomatic ITP and 3 were receiving eltrombopag when pregnancy started. Regarding safety, neither thromboembolic events among mothers nor Tpo-RA–related fetal or neonatal complications were observed, except for 1 case of neonatal thrombocytosis. Response to Tpo-RAs was achieved in 77% of cases, mostly in combination with concomitant ITP therapy (70% of responders). On the basis of these preliminary findings, temporary off-label use of Tpo-RAs for severe and/or refractory ITP during pregnancy seems safe for both mother and neonate and is likely to be helpful, especially before delivery.
Introduction
Immune thrombocytopenia (ITP) occurs in 1 to 2 per 1000 pregnancies1 ; it may precede pregnancy or be newly diagnosed during pregnancy.2,3 Except for preparation for delivery, indications for treating ITP during pregnancy are comparable to those of ITP outside pregnancy; on average, at least one-third of pregnant women with chronic ITP will require treatment during pregnancy.2,4,5 The use of corticosteroids, alone or in combination with IV immunoglobulin, is safe for both mother and fetus, and they can be used as maintenance and/or rescue treatment at any time, especially in preparation for delivery.2,4-6 Conversely, many treatments for ITP are either not recommended during pregnancy (rituximab)7 or truly contraindicated (mycophenolate mofetil, vinca alkaloid).6 Other immunosuppressors are not teratogenic, but either slow mode of action (azathioprine) or risk of hypertension (ciclosporin) limits their use.2 Lastly, data on the safety and efficacy of splenectomy during pregnancy are limited.8,9 Therefore, management of women with ITP during pregnancy with poor response to corticosteroids and IV immunoglobulin is challenging. A study from China reported the efficacy and safety of recombinant thrombopoietin (Tpo) for treating ITP in 31 pregnancies.10,11 However, the Tpo administered in this study is neither approved for ITP nor widely available outside China. Tpo receptor agonists (Tpo-RAs) eltrombopag and romiplostim, which were approved for adult ITP >10 years ago, are not recommended during pregnancy, because they are likely to cross the placenta, and their safety is not established.12 To better assess the efficacy and safety of Tpo-RAs for ITP during pregnancy, we performed a retrospective multicenter observational study.
Study design
This was an observational multicenter international study established by the French reference center for adult immune cytopenias extended to a group of international ITP experts. To be included, patients had to fulfill the following criteria: pregnant woman, age ≥18 years, diagnosis of either primary or secondary ITP,4,5 treatment with eltrombopag or romiplostim for at least 1 week for ITP during pregnancy, and at least 1 month of follow-up after Tpo-RA initiation. Women who became pregnant while on Tpo-RAs could be included as long as sufficient data on pregnancy outcome were available. All available clinical and biological data from the mothers and neonates were collected and analyzed. Treatment response was defined as complete response or response, according to standard definitions.4,5 Data are presented as mean ± standard deviation (SD) or median (interquartile range) for continuous variables, depending on distribution. Categorical variables are presented as number (percentage).
The study was performed in accordance with the ethical standards of the Helsinki Declaration and approved by our local institutional review board.
Results and discussion
In total, data from 15 women, 17 pregnancies, and 18 neonates (1 twin pregnancy) were analyzed (Table 1). Ten of 15 patients had preexisting chronic ITP (mean duration, 9.3 ± 3.2 years), including 2 cases of secondary ITP; 1 patient had persistent ITP at pregnancy onset; and ITP was diagnosed during pregnancy in 4 cases (Table 1). Median number of treatment lines before the use of Tpo-RAs was 3.0 (range, 2-7 lines), including splenectomy for 5 patients. Patients were exposed during pregnancy to either eltrombopag (n = 8; mean maximal daily dose, 60 mg [SD, ±24.4 mg]; median dose, 50 mg [range, 25-100 mg]) or romiplostim (n = 7; mean maximal weekly dose, 7.28 μg/kg [SD, ±2.97 μg/kg]; median dose, 7 μg/kg [range, 3-10 μg/kg]). Patient 15, who had severe chronic refractory ITP, was successively treated with romiplostim during a first pregnancy13 and was receiving eltrombopag when a second unexpected pregnancy occurred; eltrombopag was continued for 39 weeks until delivery and beyond, because she refused to interrupt the pregnancy. She gave birth at term to a healthy baby (weight, 2.7 kg). Median time of exposure to Tpo-RAs during pregnancy was 4.4 weeks (range, 1-39 weeks). At time of Tpo-RA initiation, ITP was symptomatic in 13 (76%) of 17 pregnancies, with mucosal bleeding in 6 cases and life-threatening gastrointestinal bleeding in 1 case (Table 1). Tpo-RAs were started beyond week 32 of gestation in preparation for delivery, because platelet count was ≤ 20 × 109/L in 10 (58%) of 17 pregnancies; in 4 cases, patients with chronic ITP were receiving Tpo-RAs when they became pregnant, and in 3 cases, Tpo-RAs were started early in the third trimester for symptomatic ITP not responding to standard therapy.
ITP characteristics and outcomes
| Patient n . | Patient age, y . | ITP phase . | N of prior treatment lines for ITP . | Tpo-RA (maximal dose during pregnancy) . | Gestation at Tpo-RA initiation, wk (treatment duration during pregnancy, wk) . | Platelet count nadir during pregnancy/platelet count before Tpo-RA/platelet count at time of delivery, × 109/L . | Bleeding manifestations/main reason for using Tpo-RA . | Pattern of initial platelet response (concomitant ITP therapy, yes or no)* . |
|---|---|---|---|---|---|---|---|---|
| 1 | 33 | Chronic | 4 (splenectomy) | Eltrombopag (50 mg) | 36 (4) | 10/10/110 | No/in preparation for delivery | CR (no) |
| 1† | 35 | Chronic | 4 (splenectomy) | Eltrombopag (25 mg) | 38 (2) | 9/15/94 | No/in preparation for delivery | CR (no) |
| 2 | 30 | Chronic | 3 | Eltrombopag (50 mg) | 8‡ (12) | 1/2/123 | Yes (skin + mucosa)/unexpected pregnancy on eltrombopag | R (yes) |
| 3 | 36 | Chronic | 2 | Eltrombopag (50 mg) | 4‡ (9) | 4/4/114 | Yes (skin)/unexpected pregnancy on eltrombopag | CR (yes) |
| 4 | 29 | Chronic | 2 | Eltrombopag (50 mg) | 33 (7) | 7/14/169 | Yes (skin + mucosa)/in preparation for delivery | CR (no) |
| 5 | 21 | Chronic | 7 (splenectomy) | Eltrombopag (75 mg) | 8‡ (12) | 50/10‡/68 | No/chronic refractory ITP, unexpected pregnancy on eltrombopag | R (no) |
| 6 | 29 | Chronic | 2 | Eltrombopag (75 mg) | 34 (5) | 8/13/23 | Yes (skin)/in preparation for delivery | NR (no) |
| 7 | 32 | Newly diagnosed | 2 | Eltrombopag (50 mg) | 27 (10) | 4/4/64 | Yes (skin)/insufficient response to steroids + IV immunoglobulin | R (no) |
| 8 | 32 | Persistent secondary ITP§ | 4 | Eltrombopag (100 mg) | 30 (4) | 1/6/136 | Yes (skin + mucosa)/refractory ITP | R (yes) |
| 9 | 33 | Chronic | 4 (splenectomy) | Romiplostim (10 μg/kg) | 39 (1) | 1/1/6 | Yes (skin)/in preparation for delivery | NR (yes) |
| 10 | 24 | Chronic | 5 (splenectomy) | Romiplostim (10 μg/kg) | 34 (6) | 12/12/250 | Yes (skin)/in preparation for delivery | CR (yes) |
| 11 | 19 | Chronic | 4 | Romiplostim (10 μg/kg) | 32 (1) | 2/15/7 | Yes (skin, mucosa)/in preparation for delivery | NR (yes) |
| 12 | 36 | Newly diagnosed | 2 | Romiplostim (7.5 μg/kg) | 37 (1) | 5/10/40 | Yes (skin + mucosa)/in preparation for delivery | CR (yes) |
| 13 | 38 | Chronic | 3 (splenectomy) | Romiplostim (3 μg/kg) | 36 (1) | 5/19/120 | Yes (skin + mucosa)/in preparation for delivery | CR (yes) |
| 14 | 29 | Newly diagnosed | 2 | Romiplostim (4 μg/kg) | 37 (1) | 17/20/21 | Yes (skin)/in preparation for delivery | CR (yes) |
| 15 | 34 | Newly diagnosed secondary ITP‖ | 7 | Romiplostim (500 μg) | 31 (3) | 3/4/367 | Yes (skin, active GI tract bleeding, hematuria)/severe refractory ITP | R (yes) |
| 15† | 37 | Chronic secondary ITP‖ | 7 | Eltrombopag (50 mg) | 36‡ (39) | 44/55/249 | No/chronic refractory secondary ITP | R (yes) |
| Patient n . | Patient age, y . | ITP phase . | N of prior treatment lines for ITP . | Tpo-RA (maximal dose during pregnancy) . | Gestation at Tpo-RA initiation, wk (treatment duration during pregnancy, wk) . | Platelet count nadir during pregnancy/platelet count before Tpo-RA/platelet count at time of delivery, × 109/L . | Bleeding manifestations/main reason for using Tpo-RA . | Pattern of initial platelet response (concomitant ITP therapy, yes or no)* . |
|---|---|---|---|---|---|---|---|---|
| 1 | 33 | Chronic | 4 (splenectomy) | Eltrombopag (50 mg) | 36 (4) | 10/10/110 | No/in preparation for delivery | CR (no) |
| 1† | 35 | Chronic | 4 (splenectomy) | Eltrombopag (25 mg) | 38 (2) | 9/15/94 | No/in preparation for delivery | CR (no) |
| 2 | 30 | Chronic | 3 | Eltrombopag (50 mg) | 8‡ (12) | 1/2/123 | Yes (skin + mucosa)/unexpected pregnancy on eltrombopag | R (yes) |
| 3 | 36 | Chronic | 2 | Eltrombopag (50 mg) | 4‡ (9) | 4/4/114 | Yes (skin)/unexpected pregnancy on eltrombopag | CR (yes) |
| 4 | 29 | Chronic | 2 | Eltrombopag (50 mg) | 33 (7) | 7/14/169 | Yes (skin + mucosa)/in preparation for delivery | CR (no) |
| 5 | 21 | Chronic | 7 (splenectomy) | Eltrombopag (75 mg) | 8‡ (12) | 50/10‡/68 | No/chronic refractory ITP, unexpected pregnancy on eltrombopag | R (no) |
| 6 | 29 | Chronic | 2 | Eltrombopag (75 mg) | 34 (5) | 8/13/23 | Yes (skin)/in preparation for delivery | NR (no) |
| 7 | 32 | Newly diagnosed | 2 | Eltrombopag (50 mg) | 27 (10) | 4/4/64 | Yes (skin)/insufficient response to steroids + IV immunoglobulin | R (no) |
| 8 | 32 | Persistent secondary ITP§ | 4 | Eltrombopag (100 mg) | 30 (4) | 1/6/136 | Yes (skin + mucosa)/refractory ITP | R (yes) |
| 9 | 33 | Chronic | 4 (splenectomy) | Romiplostim (10 μg/kg) | 39 (1) | 1/1/6 | Yes (skin)/in preparation for delivery | NR (yes) |
| 10 | 24 | Chronic | 5 (splenectomy) | Romiplostim (10 μg/kg) | 34 (6) | 12/12/250 | Yes (skin)/in preparation for delivery | CR (yes) |
| 11 | 19 | Chronic | 4 | Romiplostim (10 μg/kg) | 32 (1) | 2/15/7 | Yes (skin, mucosa)/in preparation for delivery | NR (yes) |
| 12 | 36 | Newly diagnosed | 2 | Romiplostim (7.5 μg/kg) | 37 (1) | 5/10/40 | Yes (skin + mucosa)/in preparation for delivery | CR (yes) |
| 13 | 38 | Chronic | 3 (splenectomy) | Romiplostim (3 μg/kg) | 36 (1) | 5/19/120 | Yes (skin + mucosa)/in preparation for delivery | CR (yes) |
| 14 | 29 | Newly diagnosed | 2 | Romiplostim (4 μg/kg) | 37 (1) | 17/20/21 | Yes (skin)/in preparation for delivery | CR (yes) |
| 15 | 34 | Newly diagnosed secondary ITP‖ | 7 | Romiplostim (500 μg) | 31 (3) | 3/4/367 | Yes (skin, active GI tract bleeding, hematuria)/severe refractory ITP | R (yes) |
| 15† | 37 | Chronic secondary ITP‖ | 7 | Eltrombopag (50 mg) | 36‡ (39) | 44/55/249 | No/chronic refractory secondary ITP | R (yes) |
CR, complete response; GI, gastrointestinal; NR, no response; R, response.
Includes mostly corticosteroids and/or IV immunoglobulin ± platelet transfusion.
Second pregnancy occurred in same patient.
Weeks before pregnancy (patient already receiving ELT when unplanned pregnancy occurred).
Sjögren syndrome–associated ITP.
Systemic lupus erythematosus–associated ITP with positive lupus anticoagulant, immunoglobulin G anticardiolipid antibody, and anti–β2-glycoprotein 1.
Two patients receiving Tpo-RAs reported mild headache, but no serious adverse events, especially no clinically symptomatic thromboembolic events, were observed. No patient was receiving thromboprophylaxis, including patient 15, who was triple positive for lupus anticoagulant, anticardiolipin, and anti–β2-glycoprotein 1 antibodies.
No fetal loss occurred; delivery occurred preterm (between 34 and 38 weeks of gestation) in 5 (29%) of 17 pregnancies. Mode of delivery was vaginal in 11 cases and caesarean section in 6 (all for obstetrical reasons). Platelet counts were available at birth for 14 of 18 neonates, and neonatal thrombocytopenia was found in 6, 3 of whom were neonates, including twins from 1 mother. One case of neonatal thrombocytosis (platelet count at 558 × 109/L) was observed. Birth data were available for all neonates; 2 severe neonatal complications (trisomy 8, n = 1; pulmonary artery stenosis, n = 1) leading to 1 death and 1 grade 1 intraventricular hemorrhage occurred. None of these events were directly attributable to Tpo-RA exposure in the mother (Table 2).
Neonatal complications
| Case n (mother n) . | Gestation at Tpo-RA initiation, wk (treatment duration during pregnancy, wk) . | Type of event in neonate . | Outcome (intervention) . | Causal relationship with Tpo-RA exposure in mother (yes or no)/comment . |
|---|---|---|---|---|
| 1 (1) | 36 (4) | Neonatal thrombocytopenia; platelet count, 6 × 109/L | Recovery (IV immunoglobulin + platelet transfusion) | No |
| 2 (1) | 36 (2) | Neonatal thrombocytopenia; platelet count, 8 × 109/L | Recovery (no treatment) | No |
| 3* (1) | 36 (2) | Neonatal thrombocytopenia; platelet count, 34 × 109/L | Recovery (IV immunoglobulin + platelet transfusion) | No |
| 4 (4) | 33 (7) | Neonatal thrombocytopenia; platelet count, 20 × 109/L | Recovery (IV immunoglobulin) | No |
| 5 (11) | 32 (1) | Neonatal thrombocytopenia; platelet count, 4 × 109/L | Recovery (IV immunoglobulin + platelet transfusion) | No |
| 6 (13) | 36 (1) | Neonatal thrombocytopenia; platelet count, 20 × 109/L | Recovery (IV immunoglobulin) | |
| 7 (9) | 36 (1) | Trisomy 8 (undetected preterm) | Died on day 7 | No/only 1-wk exposure to romiplostim in preparation for delivery |
| 8 (14) | 37 (1) | Pulmonary artery stenosis diagnosed during pregnancy | Favorable outcome after surgery at 2 wk of life | No/only 1-wk exposure to eltrombopag in preparation for delivery |
| 9 (8) | 30 (4) | Grade 1 intraventricular hemorrhage at birth (cranial ultrasound) | Favorable outcome without any sequela (no intervention) | Very unlikely, prematurity, favorable outcome |
| 10 (8) | 30 (4) | Neonatal thrombocytosis; platelet count, 555 × 109/L | Persistent thrombocytosis for few wk (breastfeeding on eltrombopag) | Possible |
| Case n (mother n) . | Gestation at Tpo-RA initiation, wk (treatment duration during pregnancy, wk) . | Type of event in neonate . | Outcome (intervention) . | Causal relationship with Tpo-RA exposure in mother (yes or no)/comment . |
|---|---|---|---|---|
| 1 (1) | 36 (4) | Neonatal thrombocytopenia; platelet count, 6 × 109/L | Recovery (IV immunoglobulin + platelet transfusion) | No |
| 2 (1) | 36 (2) | Neonatal thrombocytopenia; platelet count, 8 × 109/L | Recovery (no treatment) | No |
| 3* (1) | 36 (2) | Neonatal thrombocytopenia; platelet count, 34 × 109/L | Recovery (IV immunoglobulin + platelet transfusion) | No |
| 4 (4) | 33 (7) | Neonatal thrombocytopenia; platelet count, 20 × 109/L | Recovery (IV immunoglobulin) | No |
| 5 (11) | 32 (1) | Neonatal thrombocytopenia; platelet count, 4 × 109/L | Recovery (IV immunoglobulin + platelet transfusion) | No |
| 6 (13) | 36 (1) | Neonatal thrombocytopenia; platelet count, 20 × 109/L | Recovery (IV immunoglobulin) | |
| 7 (9) | 36 (1) | Trisomy 8 (undetected preterm) | Died on day 7 | No/only 1-wk exposure to romiplostim in preparation for delivery |
| 8 (14) | 37 (1) | Pulmonary artery stenosis diagnosed during pregnancy | Favorable outcome after surgery at 2 wk of life | No/only 1-wk exposure to eltrombopag in preparation for delivery |
| 9 (8) | 30 (4) | Grade 1 intraventricular hemorrhage at birth (cranial ultrasound) | Favorable outcome without any sequela (no intervention) | Very unlikely, prematurity, favorable outcome |
| 10 (8) | 30 (4) | Neonatal thrombocytosis; platelet count, 555 × 109/L | Persistent thrombocytosis for few wk (breastfeeding on eltrombopag) | Possible |
Twin of case 2.
Median platelet count at time of Tpo-RA initiation was 10 × 109/L (range, 1 × 109/L to 20 × 109/L), and an initial response was achieved in 10 (77%; complete response, n = 6; response, n = 4), whereas no response was seen in 3 patients with longstanding chronic ITP, including 2 splenectomized patients. Seven of 10 responders received concomitant ITP medication, mostly corticosteroids. Median platelet count at delivery was 94 × 109/L (range, 6 × 109/L to 250 × 109/L), with a count < 50 × 109/L in only 5 (29%) of 17 and no case of severe maternal bleeding.
Although reassuring case reports on the use of romiplostim or eltrombopag for ITP during pregnancy have been published,13-21 Tpo-RAs are not recommended in pregnancy, and experience is very limited.2,12 On the basis of the 15 patients reported here, it is reassuring that no severe adverse events and no thrombosis occurred. However, placental infarction has been previously reported,16 and Tpo-RAs during pregnancy should be used with particular caution in patients with lupus and/or antiphospholipid antibody syndrome–associated ITP.22 Interestingly, exposure to Tpo-RAs at the end of pregnancy does not seem to have a major impact on fetal thrombopoiesis, because thrombocytosis was observed in only 1 neonate, in keeping with recombinant Tpo.10 Conversely, severe neonatal thrombocytopenia occurred in 6 neonates, but 3 were from the same mother, who had previously undergone splenectomy.3,23 No correlation was found between platelet response or treatment duration in the mother and platelet count in the neonate (data not shown).
Neonatal serious complications, including 1 death, occurred in 2 of 18 neonates, neither of which could be related to Tpo-RA treatment. Efficacy of Tpo-RAs administered either alone or often in combination with another ITP therapy was in keeping with expectations, taking into account that patients had experienced failure of a median of 3 previous ITP treatment lines, including splenectomy for 5 patients. There were no obvious differences between eltrombopag and romiplostim. Limitations of this study are its uncontrolled retrospective design; the relatively small number of cases, with possible recruitment bias; and potential underestimation of placental thrombosis.
In conclusion, transient use of eltrombopag or romiplostim for pregnant women with ITP who are refractory to at least corticosteroids and IV immunoglobulin and need treatment because of bleeding manifestations, because of profound thrombocytopenia, and/or in preparation for delivery seems safe for the mother and the fetus/neonate. However, a prospective international registry is needed to monitor whether, with more widespread use of these treatments in the future, any maternal or neonatal complications may emerge, especially in the neonatal and postneonatal periods.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
The first pregnancy of patient 15 has been reported previously in Alkaabi et al.13
Authorship
Contribution: M.M. coordinated the study, included patients, and wrote the manuscript; M.R., T.J.G.-L., S.A., S.C., W.G., T.H.A.T., M.E., L.T., and J.B.B. included patients and read the manuscript; B.G. participated in the coordination of the study; and M.M., B.G., and J.B.B. participated in the writing of the manuscript.
Conflict-of-interest disclosure: M.M. has received honoraria for consultancy from Amgen, Novartis, Alexion Pharmaceuticals, Rigel, and Sanofi/Bioverativ. W.G. has received lecture fees and honoraria for participation in advisory board meetings from Novartis and Amgen. T.H.A.T. has participated in advisory boards for Alexion Pharmaceuticals, Inc., Novartis, and Ablynx. L.T. has received lecture fees and honoraria for participation in advisory board meetings from Novartis. T.J.G.-L. has received advisory board honoraria from Novartis, Amgen, and Momenta and speaker’s honoraria and research support from Novartis and Amgen. M.E. has received honoraria for participation in advisory boards from Amgen, Griffols, GlaxoSmithKline, and Novartis. S.C. has participated in advisory boards for Novartis and Amgen. J.B.B. has served on advisory boards and/or consulted for Amgen, Novartis, Dova, Rigel, UCB, Argenx, Momenta, Regeneron, RallyBio, and CSL-Behring. B.G. has served as an expert for Amgen, Novartis, Griffols, LFB, and Roche and received a research grant from Amgen. The remaining authors declare no competing financial interests.
Correspondence: Marc Michel, Hôpital Henri Mondor, 51 av. du Mal de Lattre de Tassigny, Créteil, 94010, France; e-mail: marc.michel2@aphp.fr.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal