In this issue of Blood, Myllymäki et al1 evaluated the role of pretransplant leukocyte telomere length (LTL) on survival outcomes in patients with myelodysplastic syndrome (MDS). The authors found associations between recipient short LTL and poor overall survival and high risk of nonrelapse mortality (NRM) after unrelated donor hematopoietic cell transplant (HCT).
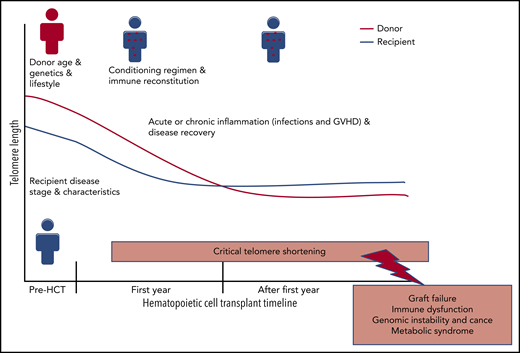
Post-HCT TL dynamics: risk factors and outcomes. GVHD, graft-versus-host disease.
Post-HCT TL dynamics: risk factors and outcomes. GVHD, graft-versus-host disease.
Telomeres are long tandem (TTAGGG)n nucleotide repeats and linked protein complexes that cap the end of chromosomes to maintain genomic stability. They shorten with each cell division and therefore are markers for cellular replicative capacity and biological aging.2 Germline pathogenic variants in telomere biology genes result in short telomere length (TL; <10th percentile for age) and cause a spectrum of illnesses called telomere biology disorders (TBDs). Patients with dyskeratosis congenita, the prototypical TBD, have a mucocutaneous phenotype and a high risk of bone marrow failure, MDS, acute myeloid leukemia, and other cancers, as well as pulmonary, liver, and other multisystem manifestations.3 Allogeneic HCT is a curative therapy for severe hematologic diseases, including MDS, but the associated mortality and morbidity risk are high. Finding biomarkers that would precisely identify patients who will benefit the most from HCT has the potential to improve patient outcomes. Recipients and donor TL have been at the center of such investigations with early studies showing significant TL attrition in transplanted donor hematopoietic stem cells in the first year after transplantation. This telomere shortening is primarily a consequence of the extensive cell proliferation needed to achieve immune reconstitution. Telomere shortening in recipient nonhematopoietic cells is also expected due to intense HCT conditioning regimens, and the graft-versus-host disease inflammatory state. Post-HCT TL shortening in donor or recipient cells may explain, at least in part, the high risk of age-related diseases in HCT recipients (reviewed in Gadalla and Savage4 ; see figure).
Myllymäki et al used the high-throughput widely used quantitative polymerase chain reaction (qPCR) assay to measure pre-HCT relative LTL and correlated it with survival outcomes in 1267 patients with MDS who received HCT at age ≥40 years. The authors showed that short LTL was associated with higher risk of NRM that corresponded with an overall survival disadvantage; this was independent of MDS somatic mutation profiles, known markers of MDS severity, or other known clinical factors affecting outcomes in those patients. The relationship between LTL and NRM, but not overall survival, was modified by the type of HCT conditioning regimen, with associations between LTL and NRM restricted to patients receiving myeloablative or fludarabine/melphalan-based conditioning regimens.1 These findings are similar to those reported in patients receiving HCT for bone marrow failure due to dyskeratosis congenita where myeloablative regimens increase patient risk of toxicity.5
Short LTL in patients with MDS can reflect markers of disease severity, such as high frequency of marrow blasts and cytogenetic abnormalities,6 or potentially identify a subset of patients with unrecognized TBDs. In Myllymäki et al, the presence of unrecognized TBD is likely because short LTL was not associated with frequency of blasts, but significant associations were noted with pre-HCT pulmonary and hepatic dysfunction. In line with that, a recent HCT study in severe aplastic anemia showed that patients with LTL < 10th percentile for age, but not those with longer telomeres, were at high risk of poor survival.7 The relationship between short LTL and NRM was previously reported in 178 patients who received HCT for different indications (13 with MDS, and 66 with acute leukemia), particularly in those with advanced malignant diseases.8 However, this association was not noted in another study of acute leukemia with complete remission (perhaps with longer LTL overall when compared with advanced disease patients).9 These studies highlight the need for large genomic investigations of aplastic anemia and myeloid neoplasms to further quantify the frequency of unrecognized TBD, overall and in patients with short telomeres. It is possible that short LTL before HCT, even in the absence of TBD, may need to be clinically managed more like a TBD rather than acquired disease.
This study adds to the accumulating recent literature suggesting a role for recipient and donor pretransplant TL in HCT prognosis. The authors acknowledged the need to use accurate TL measurement methods, such as the quantitative fluorescence in situ hybridization and flow cytometry assay for validation to address known limitations in qPCR TL assay. qPCR measures average TL in sample DNA content, as a ratio between telomeric repeat amplification to that of a single-copy gene (T/S). In the context of MDS, this method as used cannot distinguish between TL in MDS cells vs that in germline normal cells. Other assay limitations include its sensitivity to preanalytic factors such as DNA extraction method and quality of genomic DNA.9 The method is also known by its limited ability to accurately capture the shortest and longest TL, which may matter the most in the clinical setting.10
In conclusion, the use of recipient pretransplant LTL as a biomarker guiding HCT-clinical decisions is promising. Patients with LTL under certain threshold (yet to be accurately determined) may require special considerations in transplant care, such as conditioning regimen modifications and follow-up for complications associated with short telomeres (eg, metabolic diseases or subsequent cancers). The use of accurate LTL measurement methods in large studies is warranted to accomplish this goal.
Conflict-of-interest disclosure: The author declares no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal