Key Points
A new CNS-PINK model was developed and demonstrated strong ability to predict a CNS relapse in patients with ENKTL.
The ability of S-ID-MTX to prevent CNS events in high-risk CNS-PINK patients should be verified by further studies.
Abstract
Because non–anthracycline-based chemotherapy with l-asparaginase has improved survival outcomes in patients with extranodal natural killer/T-cell lymphoma (ENKTL), the incidence of central nerve system (CNS) relapse can be different when compared with that in previous reports. In this research, we sought to identify the incidence of and predictors for CNS relapse and to evaluate the necessity of CNS prophylaxis with intermediate-dose methotrexate (ID-MTX). The records of 399 patients in the training cohort and 253 patients in the validation cohort with ENKTL who received non-anthracycline–based chemotherapy were reviewed. Patients were divided into 2 groups according to whether the chemotherapy regimen included ID-MTX above 2 g/m2. A new central nervous system-prognostic index of natural killer (CNS-PINK) model was developed using 1-point powerful predictors of CNS relapse (PINK; hazard ratio [HR], 2.908; P = .030 and extranodal involvement [≥2]; HR, 4.161; P = .001) and was calculated as a sum of scores. The high-risk group of CNS-PINK was defined as 2 points. The cumulative incidence of CNS relapse was different between the CNS-PINK risk groups in the training (P < .001) and validation (P = .038) cohorts. Patients in the high-risk CNS-PINK group who were treated with SMILE or SMILE-like regimens with ID-MTX (S-ID-MTX) displayed a lower incidence rate of CNS relapse than did those who received other regimens without ID-MTX in the training cohort (P = .029). The CNS-PINK was demonstrated its strong predictability of CNS relapse in ENKTL patients. The effectiveness of S-ID-MTX in preventing CNS events in high-risk CNS-PINK patients should be verified in future studies.
Introduction
Extranodal natural killer/T-cell lymphoma (ENKTL), nasal type is a rare subtype of non-Hodgkin lymphoma characterized by extranodal involvement and Epstein-Barr virus (EBV) infection.1 The disease has a poor prognosis and is more common in Asian and Latin American countries.2 The median overall survival (OS) is ∼7.4 to 12.5 months with cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP)-based chemotherapy.3,4
Recently, non–anthracycline-based treatments, including l-asparaginase, in cooperation with radiotherapy have provided novel regimens that change the natural course of ENKTL. Radiotherapy with dexamethasone, etoposide, ifosfamide, and carboplatin (RT-DeVIC) and concurrent chemoradiotherapy with cisplatin followed by etoposide, ifosfamide, cisplatin, and dexamethasone (CCRT-VIPD) were demonstrated as effective treatments for localized ENKTL.5-8 For advanced ENKTL, improved survival outcomes have been shown with l-asparaginase–containing chemotherapies, such as corticosteroid, methotrexate (MTX), ifosfamide, l-asparaginase, and etoposide (SMILE)9 ; l-asparaginase, MTX, and dexamethasone (AspaMetDex)10 ; and gemcitabine, pegaspargase, cisplatin, and dexamethasone (DDGP).11,12
Central nervous system (CNS) involvement is rare and ranges from 0% to 6% in patients with ENKTL.13,14 Because the nasal cavity and paranasal sinus are close to the CNS across bony structures, ENKTL has the potential risk of CNS disease. However, this risk factor is masked by the relatively short survival duration. Because non–anthracycline-based chemotherapy with l-asparaginase has improved survival outcomes, the incidence of CNS relapse can be different when compared with previous reports. Kim et al13 suggested that CNS prophylaxis be considered for the high NK/T-cell lymphoma prognostic index (NKPI) group, but further evidence is needed because available studies to date have included only a small number of patients and the old CHOP-based regimens.
In this study, we investigated the incidence of and predictors for CNS relapse in patients with ENKTL and evaluated the need for using SMILE or SMILE-like regimens with intermediate-dose MTX (S-ID-MTX) as CNS prophylaxis in the era of new non–anthracycline-based treatments.
Methods
Patients and treatments in the training cohort
We reviewed 399 patients treated at either the Samsung Medical Center, Asan Medical Center, or Seoul National University Hospital in Korea from January 2000 through January 2019. We retrospectively collected data from consecutive patients diagnosed with ENKTL. Some of the patients had participated in a previous study.15 In the inclusion criteria, patients had to be pathologically confirmed to have newly diagnosed ENKTL according to the World Health Organization classification and had to have received non–anthracycline-based chemotherapy. The pathology was reviewed by designated hematopathologist in each country (ie, local center diagnosis). All cases were EBV-early transcripts–positive. Aggressive NK cell leukemia, a chronic lymphoproliferative disorder of NK cells, and other leukemias were excluded from the analysis. CNS evaluation was performed only in the presence of signs or symptoms suggesting CNS involvement. Most patients did not undergo initial CNS evaluation unless they were exhibiting neurologic symptoms. Patients with confirmed CNS involvement by examination at the time of diagnosis were excluded. CNS relapse was defined as brain parenchymal or leptomeningeal involvement confirmed by either brain magnetic resonance imaging or cerebrospinal fluid (CSF) study during chemotherapy or the follow-up period. The cerebrospinal fluid (CSF) analysis was performed by cytology or flow cytometry. The flow cytometry panel for NK/T cell lymphoma included the following antibodies: CD2, CD3, CD4, CD5, CD7, CD8, CD45, and CD56. Among the cases with leptomeningeal involvement in the training cohort, the CSF of 7 patients (46.6%) was evaluated by flow cytometry. A brain biopsy was not performed. Time to CNS relapse was defined as the time from initial diagnosis to the confirmation of CNS involvement.
Medical records were reviewed for the following characteristics: age, sex, date of diagnosis, data of death or last follow-up visit, date and status of CNS relapse, Eastern Cooperative Oncology Group performance score, B symptoms, bone marrow involvement, nonnasal type, EBV DNA, serum lactate dehydrogenase (LDH), extranodal involvement, distant lymph node involvement, Ann Arbor stage, and treatment strategy. For subgroup analysis, we used the prognostic index of natural killer (PINK) lymphoma. The parameters for this index were age >60 years, Ann Arbor stage III/IV, distant lymph node involvement, and nonnasal type.
Patients were divided into 2 groups according to whether the chemotherapy regimen included ID-MTX. An ID-MTX regimen was defined as chemotherapy including >2 g/m2 of MTX. The ID-MTX group included SMILE and MIDLE (SMILE-like regimen; dexamethasone or solumedrol, methotrexate, ifosfamide, l-asparaginase, and etoposide), whereas the group without ID-MTX included the following regimens: VIPD (etoposide, ifosfamide, cisplatin, and dexamethasone), VIDL (etoposide, ifosfamide, dexamethasone, and l-asparaginase), IMEP (ifosfamide, methotrexate, etoposide, and prednisolone), with or without l-asparaginase (or pegasparagase), and GDPL (gemcitabine, dexamethasone, cisplatin, and l-asparaginase; supplemental Table 1, available on the Blood Web site). SMILE or SMILE-like regimens with ID-MTX were abbreviated as S-ID-MTX. The most common regimen for the localized stage was VIDL (n = 133, 51.0%). SMILE (n = 64, 46.4%) was most commonly used for the advanced stage. Treatment strategies including chemotherapy regimen depended upon clinician decision-making at each center.
Patients and treatments in the validation cohort
A total of 253 patients treated at 31 institutions in Japan from 2000 through 2013 were selected from a data set of a previous study in Japan.16 The inclusion and exclusion criteria were followed as for the training cohort. Among 358 patients from the previous data set (Next-Generation Therapy for NK/T-Cell Lymphoma in East Asia [NKEA] project), 105 patients who received no treatment, radiotherapy alone, or CHOP-like chemotherapy were excluded. Chemotherapy with ID-MTX included SMILE and HyperMAIL (SMILE-like regimen; methotrexate, cytarabine, ifosfamide, l-asparaginase), and regimens without ID-MTX included DeVIC (dexamethasone, etoposide, ifosfamide, and carboplatin) and SMILE without MTX (supplemental Table 1). The most common regimen for the localized stage was DeVIC (n = 169; 86.7%), whereas l-asparaginase–containing chemotherapy (n = 30; 47.6%) was most common for the advanced stage.
This study was approved by the Institutional Review Board at each site, and the requirement for written informed consent was waived because the study was retrospective.
Statistical analysis
The distribution of variables between the 2 treatment groups was assessed using the χ2 test or Fisher’s exact test. OS and survival from CNS relapse were calculated using the Kaplan-Meier method and were compared between 2 groups with the log-rank test. A Cox proportional hazards regression model was used in univariate and multivariate analyses. The results are presented as hazard ratios (HRs) and 95% confidence intervals (CIs). All risk factors with a P < .10 in univariate analyses were included in the multivariate analysis. A Cox proportional hazards regression model with backward selection was used to identify risk factors. Patients were excluded from the analysis if any single value was missing. Cumulative incidence of CNS relapse was estimated by the reverse Kaplan-Meier method, with death events censored, and was compared between 2 groups by log-rank test. The 2-year rates for CNS relapse were reported with 95% CIs. Statistical analysis was performed using IBM SPSS Statistics for Windows, version 25.0 (IBM Corp, Armonk, NY).
Results
The characteristics of 399 patients in the training cohort are listed in Table 1. The median follow-up time for survivors was 44.0 months (range, 0.4-154.8), and the median OS of all patients was 93.7 months (95% CI, 72.524-114.952). The median age was 52 years (range, 16-92). LDH levels were elevated in 180 patients (45.1%), and EBV DNA was positive in 224 patients (56.1%). Extranodal involvement was ranked ≥2 in 108 patients (27.1%), and 83 patients (20.8%) had distant LN involvement. The Ann Arbor stage was III/IV in 138 patients (34.6%), and 225 patients (56.4%) were in the intermediate- and high-risk PINK groups. Twenty-seven patients (6.8%) experienced CNS relapse during chemotherapy or follow-up (supplemental Table 2). The OS of patients with CNS relapse was 15.1 months, which was significantly different from that of patients without CNS relapse (98.9 months; P < .001) (supplemental Figure 1A). The median time to CNS relapse was 10.1 months (range, 1.7-39.1), whereas the survival duration from CNS relapse was 3.7 months (95% CI, 1.664-5.680; supplemental Figure 1B).
Characteristics of patients with ENKTL according to chemotherapy regimen
| Characteristics . | Training (N = 399) . | Validation (N = 253) . | ||||||
|---|---|---|---|---|---|---|---|---|
| Total . | S-ID-MTX . | Total . | S-ID-MTX . | |||||
| Yes (n = 100) . | No (n = 299) . | P . | Yes (n = 29) . | No (n = 224) . | P . | |||
| Age (y) | ||||||||
| >60 | 108 (27) | 24 (24) | 84 (28) | .425 | 94 (37) | 13 (45) | 81 (36) | .416 |
| ≤60 | 291 (73) | 76 (76) | 215 (72) | 159 (63) | 16 (55) | 143 (64) | ||
| Sex | ||||||||
| Male | 249 (62) | 67 (67) | 182 (61) | .273 | 173 (68) | 13 (45) | 160 (71) | .006 |
| Female | 150 (38) | 33 (33) | 117 (39) | 80 (32) | 16 (55) | 64 (29) | ||
| ECOG PS | ||||||||
| 0-1 | 340 (85) | 74 (74) | 266 (89) | .001 | 213 (84) | 22 (76) | 191 (85) | .186 |
| 2-4 | 59 (15) | 26 (26) | 33 (11) | 40 (16) | 7 (24) | 33 (15) | ||
| B symptom | ||||||||
| Yes | 133 (33) | 56 (56) | 77 (26) | .001 | 103 (41) | 16 (55) | 87 (39) | .159 |
| No | 266 (67) | 44 (44) | 222 (74) | 145 (57) | 13 (45) | 132 (59) | ||
| NA | 0 (0) | 0 (0) | 0 (0) | 5 (2) | 0 (0) | 5 (2) | ||
| BM involvement | ||||||||
| Yes | 60 (15) | 28 (28) | 32 (11) | .001 | 19 (8) | 8 (28) | 11 (5) | .001 |
| No | 339 (85) | 72 (72) | 267 (89) | 234 (92) | 21 (72) | 213 (95) | ||
| LDH | ||||||||
| Elevated | 180 (45) | 66 (66) | 114 (38) | .001 | 100 (40) | 18 (62) | 82 (37) | .014 |
| Normal | 219 (55) | 34 (34) | 185 (62) | 153 (60) | 11 (38) | 142 (63) | ||
| EBV DNA | ||||||||
| Positive | 224 (56) | 63 (63) | 161 (54) | .166 | 69 (27) | 14 (48) | 55 (25) | .259 |
| Negative | 160 (40) | 35 (35) | 125 (42) | 30 (12) | 3 (10) | 27 (12) | ||
| NA | 15 (4) | 2 (2) | 13 (4) | 154 (61) | 12 (41) | 142 (63) | ||
| Extranodal involvement | ||||||||
| ≥2 | 108 (27) | 33 (33) | 75 (25) | .123 | 64 (25) | 20 (69) | 44 (20) | .001 |
| <2 | 291 (73) | 67 (67) | 224 (75) | 189 (75) | 9 (31) | 180 (80) | ||
| Distant LN involvement | ||||||||
| Yes | 83 (21) | 38 (38) | 45 (15) | .001 | 20 (8) | 8 (28) | 12 (5) | .001 |
| No | 316 (79) | 62 (62) | 254 (85) | 233 (92) | 21 (72) | 212 (95) | ||
| Nonnasal type | ||||||||
| Yes | 64 (16.0) | 35 (35.0) | 29 (9.7) | .001 | 22 (9) | 6 (21) | 16 (7) | .027 |
| No | 335 (84.0) | 65 (65.0) | 270 (90.3) | 231 (91) | 23 (79) | 208 (93) | ||
| Ann Arbor stage | ||||||||
| I/II | 261 (65) | 34 (34) | 227 (76) | .001 | 194 (77) | 5 (17) | 189 (84) | .001 |
| III/IV | 138 (35) | 66 (66) | 72 (24) | 59 (23) | 24 (83) | 35 (16) | ||
| IPI | ||||||||
| Low | 226 (57) | 32 (32) | 194 (65) | .001 | 166 (66) | 6 (21) | 160 (71) | .001 |
| Intermediate | 138 (35) | 52 (52) | 86 (29) | 53 (21) | 13 (45) | 40 (18) | ||
| High | 35 (9) | 16 (16) | 19 (6) | 34 (13) | 10 (34) | 24 (11) | ||
| PINK | ||||||||
| Low | 174 (44) | 18 (18) | 156 (52) | .001 | 124 (49) | 2 (7) | 122 (54) | .001 |
| Intermediate | 116 (29) | 30 (30) | 86 (29) | 80 (32) | 9 (31) | 71 (32) | ||
| High | 109 (27) | 52 (52) | 57 (19) | 49 (19) | 18 (62) | 31 (14) | ||
| CCRT | ||||||||
| Yes | 211 (53) | 18 (18) | 193 (65) | .001 | 177 (70) | 1 (3) | 176 (79) | .001 |
| No | 188 (47) | 82 (82) | 106 (36) | 76 (30) | 28 (97) | 48 (21) | ||
| l-asparaginase | ||||||||
| Yes | 310 (78) | 100 (100) | 210 (70) | .001 | 33 (13) | 29 (100) | 4 (2) | .001 |
| No | 89 (22) | 0 (0) | 89 (30) | 220 (87) | 0 (0) | 220 (98) | ||
| CNS relapse | ||||||||
| Yes | 27 (7) | 5 (5) | 22 (7) | .416 | 18 (7) | 2 (7) | 16 (7) | .99 |
| No | 372 (93) | 95 (95) | 277 (93) | 235 (93) | 27 (93) | 208 (93) | ||
| Relapse type | ||||||||
| Parenchymal | 10 | 3 | 7 | 5 | 0 | 5 | — | |
| Leptomeningeal | 11 | 0 | 11 | 4 | 1 | 3 | ||
| Both | 6 | 2 | 4 | 1 | 0 | 1 | ||
| NA | 0 | 0 | 0 | 8 | 1 | 7 | ||
| Characteristics . | Training (N = 399) . | Validation (N = 253) . | ||||||
|---|---|---|---|---|---|---|---|---|
| Total . | S-ID-MTX . | Total . | S-ID-MTX . | |||||
| Yes (n = 100) . | No (n = 299) . | P . | Yes (n = 29) . | No (n = 224) . | P . | |||
| Age (y) | ||||||||
| >60 | 108 (27) | 24 (24) | 84 (28) | .425 | 94 (37) | 13 (45) | 81 (36) | .416 |
| ≤60 | 291 (73) | 76 (76) | 215 (72) | 159 (63) | 16 (55) | 143 (64) | ||
| Sex | ||||||||
| Male | 249 (62) | 67 (67) | 182 (61) | .273 | 173 (68) | 13 (45) | 160 (71) | .006 |
| Female | 150 (38) | 33 (33) | 117 (39) | 80 (32) | 16 (55) | 64 (29) | ||
| ECOG PS | ||||||||
| 0-1 | 340 (85) | 74 (74) | 266 (89) | .001 | 213 (84) | 22 (76) | 191 (85) | .186 |
| 2-4 | 59 (15) | 26 (26) | 33 (11) | 40 (16) | 7 (24) | 33 (15) | ||
| B symptom | ||||||||
| Yes | 133 (33) | 56 (56) | 77 (26) | .001 | 103 (41) | 16 (55) | 87 (39) | .159 |
| No | 266 (67) | 44 (44) | 222 (74) | 145 (57) | 13 (45) | 132 (59) | ||
| NA | 0 (0) | 0 (0) | 0 (0) | 5 (2) | 0 (0) | 5 (2) | ||
| BM involvement | ||||||||
| Yes | 60 (15) | 28 (28) | 32 (11) | .001 | 19 (8) | 8 (28) | 11 (5) | .001 |
| No | 339 (85) | 72 (72) | 267 (89) | 234 (92) | 21 (72) | 213 (95) | ||
| LDH | ||||||||
| Elevated | 180 (45) | 66 (66) | 114 (38) | .001 | 100 (40) | 18 (62) | 82 (37) | .014 |
| Normal | 219 (55) | 34 (34) | 185 (62) | 153 (60) | 11 (38) | 142 (63) | ||
| EBV DNA | ||||||||
| Positive | 224 (56) | 63 (63) | 161 (54) | .166 | 69 (27) | 14 (48) | 55 (25) | .259 |
| Negative | 160 (40) | 35 (35) | 125 (42) | 30 (12) | 3 (10) | 27 (12) | ||
| NA | 15 (4) | 2 (2) | 13 (4) | 154 (61) | 12 (41) | 142 (63) | ||
| Extranodal involvement | ||||||||
| ≥2 | 108 (27) | 33 (33) | 75 (25) | .123 | 64 (25) | 20 (69) | 44 (20) | .001 |
| <2 | 291 (73) | 67 (67) | 224 (75) | 189 (75) | 9 (31) | 180 (80) | ||
| Distant LN involvement | ||||||||
| Yes | 83 (21) | 38 (38) | 45 (15) | .001 | 20 (8) | 8 (28) | 12 (5) | .001 |
| No | 316 (79) | 62 (62) | 254 (85) | 233 (92) | 21 (72) | 212 (95) | ||
| Nonnasal type | ||||||||
| Yes | 64 (16.0) | 35 (35.0) | 29 (9.7) | .001 | 22 (9) | 6 (21) | 16 (7) | .027 |
| No | 335 (84.0) | 65 (65.0) | 270 (90.3) | 231 (91) | 23 (79) | 208 (93) | ||
| Ann Arbor stage | ||||||||
| I/II | 261 (65) | 34 (34) | 227 (76) | .001 | 194 (77) | 5 (17) | 189 (84) | .001 |
| III/IV | 138 (35) | 66 (66) | 72 (24) | 59 (23) | 24 (83) | 35 (16) | ||
| IPI | ||||||||
| Low | 226 (57) | 32 (32) | 194 (65) | .001 | 166 (66) | 6 (21) | 160 (71) | .001 |
| Intermediate | 138 (35) | 52 (52) | 86 (29) | 53 (21) | 13 (45) | 40 (18) | ||
| High | 35 (9) | 16 (16) | 19 (6) | 34 (13) | 10 (34) | 24 (11) | ||
| PINK | ||||||||
| Low | 174 (44) | 18 (18) | 156 (52) | .001 | 124 (49) | 2 (7) | 122 (54) | .001 |
| Intermediate | 116 (29) | 30 (30) | 86 (29) | 80 (32) | 9 (31) | 71 (32) | ||
| High | 109 (27) | 52 (52) | 57 (19) | 49 (19) | 18 (62) | 31 (14) | ||
| CCRT | ||||||||
| Yes | 211 (53) | 18 (18) | 193 (65) | .001 | 177 (70) | 1 (3) | 176 (79) | .001 |
| No | 188 (47) | 82 (82) | 106 (36) | 76 (30) | 28 (97) | 48 (21) | ||
| l-asparaginase | ||||||||
| Yes | 310 (78) | 100 (100) | 210 (70) | .001 | 33 (13) | 29 (100) | 4 (2) | .001 |
| No | 89 (22) | 0 (0) | 89 (30) | 220 (87) | 0 (0) | 220 (98) | ||
| CNS relapse | ||||||||
| Yes | 27 (7) | 5 (5) | 22 (7) | .416 | 18 (7) | 2 (7) | 16 (7) | .99 |
| No | 372 (93) | 95 (95) | 277 (93) | 235 (93) | 27 (93) | 208 (93) | ||
| Relapse type | ||||||||
| Parenchymal | 10 | 3 | 7 | 5 | 0 | 5 | — | |
| Leptomeningeal | 11 | 0 | 11 | 4 | 1 | 3 | ||
| Both | 6 | 2 | 4 | 1 | 0 | 1 | ||
| NA | 0 | 0 | 0 | 8 | 1 | 7 | ||
S-ID-MTX, SMILE or SMILE-like regimens with intermediate-dose methotrexate; ECOG PS, Eastern Cooperative Oncology Group performance score; BM, bone marrow; NA, not available; LN, lymph node; CCRT, concurrent chemoradiotherapy.
The characteristics of 253 patients in the validation cohort are also summarized in Table 1. Extranodal involvement was ≥2 in 64 patients (25.3%), and 129 patients (51.0%) were in the intermediate- and high-risk PINK groups. Eighteen patients (7.1%) experienced CNS relapse during chemotherapy or follow-up.
Development and validation of the CNS-PINK
In univariate analyses, LDH (HR, 2.762; 95% CI, 1.279-5.962; P = .010), EBV DNA (HR, 3.199; 95% CI, 1.282-7.982; P = .013), extranodal involvement ≥2 (HR, 7.123; 95% CI, 3.246-15.629; P < .001), distant LN involvement (HR, 4.413; 95% CI, 2.040-9.549; P < .001), Ann Arbor stage III/IV (HR, 6.665; 95% CI, 2.977-14.924; P < .001), and intermediate/high PINK (HR, 5.056; 95% CI, 1.908-13.397; P = .001) were significantly associated with a high risk of CNS relapse (Table 2). Multivariate analyses revealed extranodal involvement ≥2 (HR, 4.628; 95% CI, 1.974-10.852; P = .001) and PINK (HR, 2.677; 95% CI, 0.936-7.652; P = .066) as powerful predictors of CNS relapse (Table 2; Figure 1).
Cox regression univariate and multivariate analyses of risk factors for CNS relapse
| . | HR (95% CI) . | P . |
|---|---|---|
| Univariate analysis | ||
| Age >60 y | 1.130 (0.477-2.675) | .781 |
| LDH | 2.762 (1.279-5.962) | .010 |
| EBV DNA | 3.199 (1.282-7.982) | .013 |
| Extranodal involvement ≥2 | 7.123 (3.246-15.629) | .001 |
| Distant LN involvement | 4.413 (2.040-9.549) | .001 |
| Ann Arbor stage III/IV | 6.665 (2.977-14.924) | .001 |
| PINK | ||
| Intermediate vs low | 3.717 (1.268-10.897) | .071 |
| High vs low | 7.288 (2.553-20.807) | .001 |
| High vs intermediate | 1.932 (0.832-4.484) | .125 |
| Intermediate/high vs low | 5.056 (1.908-13.397) | .001 |
| Multivariate analysis | ||
| Extranodal involvement ≥2 | 4.628 (1.974-10.852) | .001 |
| PINK intermediate/high | 2.677 (0.936-7.652) | .066 |
| . | HR (95% CI) . | P . |
|---|---|---|
| Univariate analysis | ||
| Age >60 y | 1.130 (0.477-2.675) | .781 |
| LDH | 2.762 (1.279-5.962) | .010 |
| EBV DNA | 3.199 (1.282-7.982) | .013 |
| Extranodal involvement ≥2 | 7.123 (3.246-15.629) | .001 |
| Distant LN involvement | 4.413 (2.040-9.549) | .001 |
| Ann Arbor stage III/IV | 6.665 (2.977-14.924) | .001 |
| PINK | ||
| Intermediate vs low | 3.717 (1.268-10.897) | .071 |
| High vs low | 7.288 (2.553-20.807) | .001 |
| High vs intermediate | 1.932 (0.832-4.484) | .125 |
| Intermediate/high vs low | 5.056 (1.908-13.397) | .001 |
| Multivariate analysis | ||
| Extranodal involvement ≥2 | 4.628 (1.974-10.852) | .001 |
| PINK intermediate/high | 2.677 (0.936-7.652) | .066 |
BM, bone marrow; LN, lymph node.
Cumulative incidence of CNS relapse in patients with ENKTL. Frequency of relapse by extranodal lymph node (LN) involvement (A) and PINK risk group (B) in the training cohort.
Cumulative incidence of CNS relapse in patients with ENKTL. Frequency of relapse by extranodal lymph node (LN) involvement (A) and PINK risk group (B) in the training cohort.
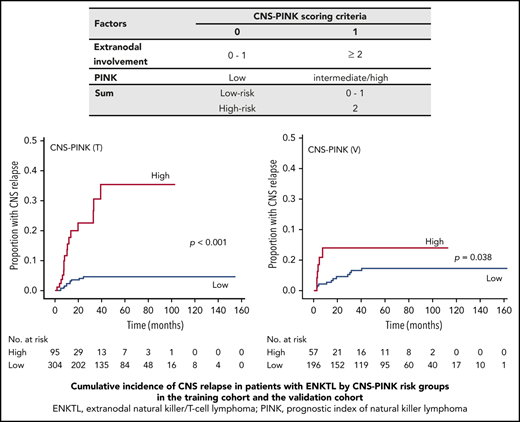
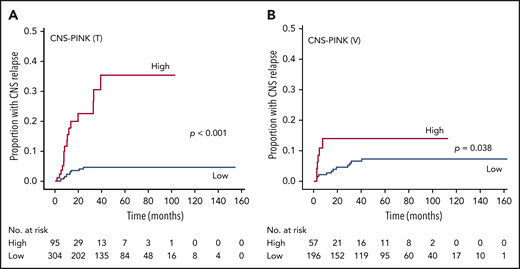
Therefore, a new prognostic model defining the CNS-PINK was developed based on extranodal involvement and PINK. Each factor was given a score of 1 point, and the CNS-PINK was calculated as a sum of scores (Table 3). Then, we divided patients into 2 risk groups: a low-risk group with 0 or 1 point (n = 304; 76.2%) vs a high-risk group with 2 points (n = 95; 23.8%). The 2-year rates for CNS relapse were 4.1% (95% CI, 1.565-6.54) for the low-risk group and 22.8% (95% CI, 10.5-33.4) for the high-risk group. The cumulative incidence of CNS relapse was significantly different between the CNS-PINK risk groups in the training cohort (P < .001; Figure 2A).
CNS-PINK scoring criteria
| Factors . | CNS-PINK scoring criteria . | |
|---|---|---|
| 0 . | 1 . | |
| Extranodal involvement | 0-1 | ≥2 |
| PINK | Low | Intermediate/high |
| Sum | Low risk | 0-1 |
| High risk | 2 | |
| Factors . | CNS-PINK scoring criteria . | |
|---|---|---|
| 0 . | 1 . | |
| Extranodal involvement | 0-1 | ≥2 |
| PINK | Low | Intermediate/high |
| Sum | Low risk | 0-1 |
| High risk | 2 | |
Cumulative incidence of CNS relapse in patients with ENKTL by CNS-PINK risk groups. Frequency in the training cohort (A) and the validation cohort (B).
Cumulative incidence of CNS relapse in patients with ENKTL by CNS-PINK risk groups. Frequency in the training cohort (A) and the validation cohort (B).
To validate the generalizability of this CNS risk model, we applied the CNS-PINK to 253 patients with ENKTL from a Japanese cohort. This cohort included 196 patients (77.5%) in the low-risk group and 57 patients (22.5%) in the high-risk group. The 2-year rates for CNS relapse were 4.5% (95% CI, 2.3-8.8) in the low-risk group and 13.9% (95% CI, 6.4-28.8) in the high-risk group. Like the result of the training cohort, the cumulative incidence of CNS relapse was significantly different between the CNS-PINK risk groups in the validation cohort (P = .038; Figure 2B).
Effects of S-ID-MTX on CNS relapse in the high-risk CNS-PINK group
We evaluated the effect of S-ID-MTX on the risk of CNS relapse. In the training cohort, S-ID-MTX included MIDLE (n = 17 patients; 17.0%) and SMILE (n = 83 patients; 83.0%), whereas regimens without ID-MTX included VIPD (n = 71 patients; 23.7%), VIDL (n = 172 patients; 57.5%), IMEP with or without l-asparaginase (n = 51 patients; 17.1%), and GDPL (n = 5 patients; 1.7%). The incidence of CNS relapse in patients with or without S-ID-MTX was 5 patients (5 of 100; 5.0%) and 22 patients (22 of 299; 7.4%), respectively.
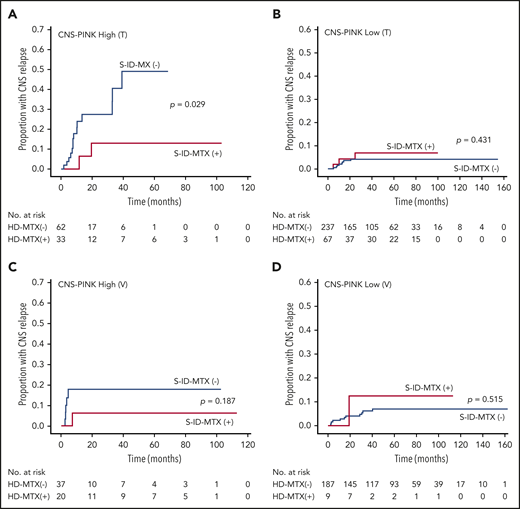
However, the cumulative incidence of CNS relapse was not different regardless of whether or not patients in the training cohort received S-ID-MTX. Therefore, we used the CNS-PINK for a risk-adapted approach. In the high- and low-risk groups of CNS-PINK, the cumulative incidence of CNS relapse was compared between treatment groups. Patients in the high-risk group who received S-ID-MTX displayed a significantly lower incidence rate of CNS relapse than did those who received other regimens (P = .029; Figure 3A). However, there were no significant differences between the treatment groups among low-risk patients (P = .431; Figure 3B).
Cumulative incidence of CNS relapse in patients with ENKTL by S-ID-MTX and CNS-PINK risk groups. Frequency in the training cohort (A-B) and the validation cohort (C-D). S-ID-MTX, SMILE or SMILE-like regimens with intermediate-dose MTX.
Cumulative incidence of CNS relapse in patients with ENKTL by S-ID-MTX and CNS-PINK risk groups. Frequency in the training cohort (A-B) and the validation cohort (C-D). S-ID-MTX, SMILE or SMILE-like regimens with intermediate-dose MTX.
We validated whether S-ID-MTX in the high-risk CNS-PINK decreased CNS relapse in the Japanese cohort. In this cohort, S-ID-MTX included SMILE (n = 26; 89.7%) and HyperMAIL (n = 3; 10.3%), whereas regimens without ID-MTX included DeVIC (n = 220; 98.2%) and SMILE without MTX (n = 4; 1.8%; Table 1; supplemental Table 1). The incidence of CNS relapse in patients with or without S-ID-MTX was 2 patients (2 of 29; 6.9%) and 16 patients (16 of 224; 7.1%), respectively. Although there were no significant differences between the treatment groups in the low-risk (P = .515) and high-risk (P = .187) CNS-PINK groups, the tendency toward reduction of the cumulative incidence of CNS relapse in the high-risk CNS-PINK group with S-ID-MTX was confirmed by the validation cohort (Figures 3C-D).
Discussion
CNS relapse in T-cell lymphoma has rarely been studied because of its low incidence. In a large population-based cohort of patients with peripheral T-cell lymphoma, 28 (4.5%) of 625 patients experienced CNS disease over time. This cohort included 26 patients with ENKTL who did not experience CNS relapse.17 A multicenter review of Asian countries reported CNS relapse in 12 (5.8%) of 208 patients with ENKTL.13 However, the median follow-up was 11.62 months and only 60 patients were treated with non–anthracycline-based chemotherapy. In our study, during 44.0 months of median follow-up, 27 (6.8%) of 399 patients with ENKTL developed CNS relapse. All of these patients were treated with non–anthracycline-based chemotherapy with or without l-asparaginase. The incidence of CNS relapse in ENKTL has increased as survival has improved with a paradigm shift in chemotherapy.
Several studies have demonstrated that CNS involvement leads to a poor prognosis in non-Hodgkin lymphoma.18-20 In a 2009 study, Kim et al13 analyzed 208 patients with ENKTL and reported a 6.03-month median time to CNS relapse (95% CI, 5.23-6.83) and a 2.53-month median OS after CNS relapse (95% CI, 0.57-4.49). Because of the development of new non–anthracycline-based treatments, we describe a considerably prolonged time to CNS relapse (15.1 months; 95% CI, 8.698-21.466) and improvement in survival duration after CNS relapse (3.7 months; 95% CI, 1.664-5.680) in the training cohort, even compared with diffuse large B-cell lymphoma (6.7 and 2.8 months in the British Columbia Cancer Agency cohort21 ). However, CNS involvement is still associated with a dismal prognosis of less than 4 months of OS after CNS relapse, regardless of lymphoma type. Therefore, better initial chemotherapy in combination with CNS prophylaxis is needed to decrease the incidence of relapse in both systemic disease and CNS involvement.
We demonstrated meaningful factors for CNS relapse, including LDH, EBV DNA, extranodal involvement, distant LN involvement, Ann Arbor stage III/IV, and PINK. Another study for ENKTL revealed that lymph node involvement (P = .006), the primary site of involvement (extra–upper aerodigestive tract NKTL; P = .008), Ann Arbor stage III/IV (P < .001) and advanced NKPI risk (group III/IV; P = .003) increased the risk of CNS disease.13 New prognostic index for ENKTL, such as PINK, were used in this study because the international prognostic index (IPI) and NKPI were developed based on anthracycline-based chemotherapy. Ellin et al17 suggested that involvement of the skin and gastrointestinal tract identifies patients with a higher risk for CNS disease in peripheral T-cell lymphoma . In ENKTL, PINK includes nonnasal-type disease. PINK was demonstrated to be a good predictor of CNS relapse in multivariate analysis.
We developed a simple and strong CNS risk model and validated it in a relatively large and independent cohort. CNS-PINK includes ≥2 extranodal lymph node involvement as one powerful factor. For example, nasal cavity and ≥1 extranodal involvement meets this factor, and ≥2 separate lesions in the same extranodal organ is also included. This factor could identify the high-risk CNS patients who may not be identified by PINK alone. CNS-IPI was developed for diffuse large B-cell lymphoma treated with R-CHOP in 2016 by Schmitz et al.21 This model consists of 6 points: a new factor of kidney and adrenal gland involvement plus 5 factors of the IPI. In contrast, CNS-PINK uses PINK as a 1-point factor, and this simplicity makes the CNS-PINK model easy to use.
Preventing CNS relapse has become important because of its extremely poor prognosis, but the role of CNS prophylaxis has never been studied in ENKTL. In this study, we planned a multicenter retrospective analysis in which patients with ENKTL were divided into 2 treatment groups according to whether their chemotherapy regimen included ID-MTX as IV CNS prophylaxis. The cumulative incidence of CNS relapse was then compared among the groups. However, no difference was observed between these 2 treatment groups in all patients of the training cohort. For a risk-adapted approach, we used a newly developed index, CNS-PINK, consisting of potent risk factors that we verified via multivariate analysis. As expected, patients in the high-risk CNS-PINK group who received S-ID-MTX showed lower incidence rates of CNS relapse than those who received other regimens in the training cohort. Because this result could not be fully verified in the validation cohort, further studies are needed for confirming the CNS effect of S-ID-MTX in high-risk CNS-PINK patients.
The optimal chemotherapy regimens for advanced ENKTL have not been established. Yang et al22 revealed that patients receiving SMILE exhibited improved OS and progression-free survival and better complete response and overall response rate than patients receiving CHOP-based regimens in stage IV relapsed or refractory ENKTL. A recent randomized, controlled, multicenter study comparing the DDGP (dexamethasone, cisplatin, gemcitabine, and pegaspargase) regimen with the SMILE regimen in newly diagnosed advanced ENKTL was conducted in China, and Wang et al23 demonstrated that the DDGP regimen produced better survival and safety than the SMILE regimen (56.6% vs 41.8% for 3-year progression-free survival; P = .004; 74.3% vs 51.7% for 5-year OS; P = .02). However, this study used the modified Ann Arbor staging system, defined localized disease as stage III, and enrolled a considerable number of patients with stage III disease. Considering the observed toxicity and tolerance, the SMILE regimen should be applied in selected patients with high-risk and advanced-stage disease. Likewise, we focused on CNS events and suggest that S-ID-MTX be considered in the high-risk CNS-PINK group. However, this strategy should be confirmed in future studies that include other regimens.
There were several limitations to this study. First, its retrospective nature, the small size of the cohort, and the corresponding small number of cases of CNS relapse may have led to bias that could weaken the results. Second, heterogeneity in chemotherapy regimens may have contributed to the relative variation in our findings. Also, the inclusion of other drugs in regimens could affect the individual protective effects of MTX. CNS-protective effects may be attributable to intermediate-dose MTX and/or other CNS-penetrating drugs such as ifosfamide, Ara-C, and etoposide in S-ID-MTX. Third, although there was a tendency toward reducing the cumulative incidence of CNS relapse, there were no statistically significant findings in the validation cohort. Our results should be validated by a prospective, large-scale randomized study in cohorts receiving other regimens. Nevertheless, this study provides useful information in this field and suggests directions for future research on CNS involvement of ENKTL.
In summary, we developed a CNS-PINK model and demonstrated its strong predictability of CNS relapse in patients with ENKTL. The effect of S-ID-MTX for preventing CNS events in high-risk CNS-PINK patients should be verified by future studies.
Original data may be obtained by e-mail request to the corresponding author.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: W.S.K. conceived and designed the study; H.K., H.J., M.Y., S.E.Y., S.B., J.Y.H., Y.K., S.-S.Y., E.J.K., M.O., K.M., S.T., D.H.Y., J.C., Y.H.K., S.J.K., R.S., and W.S.K. acquired the data; H.K., H.J., M.Y., I.S., Y.K., E.J.K., D.H.Y., R.S., and W.S.K. analyzed and interpreted the data; H.K., H.J., and W.S.K. wrote the first draft of the manuscript; and all authors contributed to reviewing or revising the manuscript and approved the final version.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Won Seog Kim, Division of Hematology-Oncology, Department of Medicine, Samsung Medical Center, Sungkyunkwan University School of Medicine, 81 Irwon-ro, Gangnam-gu, Seoul 06351, South Korea; e-mail: wskimsmc@skku.edu.
REFERENCES
Author notes
H.K. and H.J. contributed equally to this study.