In this issue of Blood, Perreault et al showed decreasing total antibody levels over time in 15 repeat COVID-19 convalescent plasma (CCP) donors with at least 4 donations.1 The decrease was greatest between the final 2 time points reported, from days 70 to 84 to days 85 to 114, after disease onset. This decrease was time dependent and independent of donation number.
Collecting and transfusing high-titer CCP units early in hospitalization improves outcome. Studies have demonstrated that antibody levels are highest when donors are first eligible to donate. The high-titer CCP units when given soon after diagnosis or hospitalization improve patient outcomes.
Collecting and transfusing high-titer CCP units early in hospitalization improves outcome. Studies have demonstrated that antibody levels are highest when donors are first eligible to donate. The high-titer CCP units when given soon after diagnosis or hospitalization improve patient outcomes.
Falling antibody levels after recovery disease are well documented, including in COVID-19 patients. Wang et al followed neutralizing antibody titers in hospitalized patients starting shortly after symptom onset and demonstrated antibodies peaked at 4 to 5 weeks after disease onset and then declined.2 In the Perreault et al study, the earliest antibody levels were obtained 33 to 53 days after disease onset as their study population was plasma donors, who were ≥14 days symptom free and never hospitalized. Muecksch et al used neutralizing antibody assays to show similar antibody titer decline in individuals who were weeks after diagnosis, but this decline was less apparent and highly variable when measuring antibody levels using high-throughput assays.3 Most recently, Gudbjartsson et al followed antibody levels in 48 hospitalized patients for ∼100 days after diagnosis and found that these levels change depending on the antibody tests: levels increasing in total immunoglobulin assays, slightly decreasing in immunoglobulin G (IgG) assays, and greatly decreasing in IgM and IgA assays.4 Thus antibody level changes during the few months after diagnosis depend on the assay.
The study by Perreault et al has important limitations. In addition to the small study population, the study used 1 enzyme-linked immunosorbent assay (ELISA) to measure severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) spike protein receptor binding domain (RBD) total (IgM/IgA/IgG) immunoglobulin from ∼30 to 100 days after symptoms. Thus, the rise and potentially continued fall of antibody levels are missing. Other studies end follow-up at a similar time point. Accordingly, studies that continue to follow individuals past a few months are needed. Finally, the assay used measured immunoglobulin titers to qualify donors with a cutoff of ≥100. These CCP donations are part of a multicenter randomized clinical trial, CONCOR-1 (https://clinicaltrials.gov/ct2/show/NCT04348656), which compares adults with COVID-19 respiratory illness treated with 500 mL of CCP to standard of care. Further CCP characterization, including comparison of the ELISA measurements with neutralization antibody titers, and its correlation with patient outcome are planned.
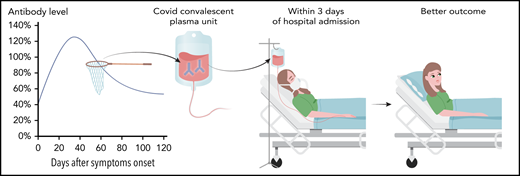
Emerging literature shows a correlation between CCP antibody level and patient outcome (see figure). Recently, outcome data from the US Expanded Access Protocol, which has enrolled >105 000 patients and transfused >75 000 units, were made available.5 Patients treated early after diagnosis (within 3 days) with CCP containing high levels of IgG against the SARS-CoV-2 spike subunit 1 protein (signal-to-cutoff ratio [S/CO] >18.45 Ortho VITROS SARS-CoV-2 IgG test) had better outcomes than those who received CCP with lower-antibody levels and/or longer after diagnosis. Similarly, data from Houston Methodist Hospital showed earlier transfusion (≤72 hours from admission) with higher-titer CCP (≥1350 using ELISA against anti-RBD IgG) resulted in decreased 28-day mortality compared with patients who received lower-titer CCP after 72 hours from admission (2% vs 20%).6 In both of these studies, earlier transfusion appears more significant than CCP antibody titers on outcome.
Other studies support that the combination of antibody levels and timing of transfusion is important for outcome. Xia et al demonstrated higher-antibody level in units correlated with higher-antibody level increase in recipients and resulted in better clinical response.7 In addition, as patients develop a measurable humoral response ∼10 to 12 days after exposure, shortly after symptom onset, transfusion of CCP prior to antibody formation or when antibody levels are still low is logical.
These positive clinical results plus historic use of CCP in other respiratory coronaviruses, preclinical data, and additional studies of safety and efficacy supported the Food and Drug Administration (FDA) issuing emergency use authorization (EUA) for CCP.8 The EUA recommends labeling the CCP with high- or low-antibody titer, however, is silent regarding earlier treatment. The FDA recommends that units with S/CO of ≥12 on the Ortho assay are “High Titer.”8 All units with S/CO <12 must be labeled as “Low Titer.” The FDA has recommended that blood centers wishing to use a different antibody platform must demonstrate equivalence to the Ortho VITROS S/CO of ≥12 in order to define high-titer units. Given this S/CO is in the middle range of CCP units and below the S/CO with the best patient outcomes, patients may not benefit as much as if units had been labeled with the numerical titer equivalent or if the cutoff had been made at the level where outcomes were most improved.5
Another issue is hospitalized patients who received CCP are deferred from donating for 3 months. This deferral applies to all transfused patients as well as other activities deemed HIV risk. This time period coincides with the greatest rate of antibody decline shown by Perreault et al, which could result in fewer donors meeting the high-titer criteria. However, given the large variability of donor antibody titers, those with high levels should still be eligible to donate high-titer plasma. In addition, low-titer plasma is still able to be transfused, but the treating physician may then need to give more units to ensure an adequate antibody dose. However, without labeling the units with the titer equivalent, understanding how many and which units to transfuse is difficult.
Effective CCP should contain antibody levels that correlate with measured improvements in patient outcome. High-titer CCP issued under the EUA regulations moves toward this goal, but based on current data, has fallen short. Further studies are needed to determine the necessary antibody level to achieve optimal patient outcomes. The study by Perreault et al reiterated the interindividual variability in antibody levels as well as the declining antibody levels over time. Given these results and the new rules defined by the EUA, achieving a sufficient inventory of high-titer CCP may be difficult. Since higher-antibody levels correlate with disease severity, hospitals should encourage specific patient populations to donate, while blood centers should develop processes to identify high-titer donors and take measures to encourage multiple repeat donations. Most importantly, these products need to be given early in the hospital/disease course in order to have optimal therapeutic benefit.
Conflict-of-interest disclosure: The authors declare no competing financial interests.