In this issue of Blood, Grey et al have shown that stimulation of the receptor tyrosine kinase RET allows for significant expansion of cord blood hematopoietic stem cells (HSCs).1
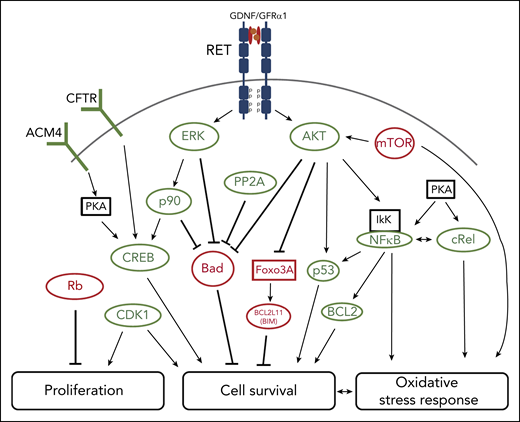
GDNF/GFRα1 induces an array of downstream responses upon associating with the RET receptor in cultured cord blood HSCs. Illustrated pathways were identified through a combination of kinome profiling, mass cytometry, and assessment of RNA level changes. Activating (green) and inhibiting (red) phosphorylations, protein levels or RNA levels, and proposed modes of action are shown. Altogether, RET stimulation promotes a coordinated program of increased HSC proliferation, survival, and resistance to oxidative stress to potentiate HSC expansion. See Figure 5E in the article by Grey et al that begins on page 2535.
GDNF/GFRα1 induces an array of downstream responses upon associating with the RET receptor in cultured cord blood HSCs. Illustrated pathways were identified through a combination of kinome profiling, mass cytometry, and assessment of RNA level changes. Activating (green) and inhibiting (red) phosphorylations, protein levels or RNA levels, and proposed modes of action are shown. Altogether, RET stimulation promotes a coordinated program of increased HSC proliferation, survival, and resistance to oxidative stress to potentiate HSC expansion. See Figure 5E in the article by Grey et al that begins on page 2535.
The ability of HSCs to regenerate a complete and lasting hematopoietic system underpins the effectiveness of HSC transplantation, which provides lifesaving treatment of a multitude of malignant hematopoietic diseases and immune disorders. Unfortunately, allogeneic HSC transplantation as a treatment option is often precluded by the inability to find a matched bone marrow or mobilized peripheral blood donor. Cord blood HSCs represent a potential solution given their ready availability and reduced propensity to induce graft-versus-host disease. However, the number of HSCs per cord product remains an obstacle for adult transplantation.2 Moreover, the amplification of HSC numbers prior to transplant has been difficult due to their rapid differentiation and loss in culture. This same limitation also presents a critical challenge for emerging gene therapy strategies that aim to capitalize on state-of-the-art genome engineering tools to tailor HSCs in the dish prior to transplantation. To address these issues, identifying agents that promote ex vivo HSCs expansion is a logical approach. Although there have been recent advances, most notably the AHR antagonist SR1 and the small-molecule UM171, improvements that could allow for HSC expansion with limited exhaustion will require a more complete accounting of the extrinsic inputs and intrinsic programs HSCs capitalize on to ensure their self-renewal.3,4
Nervous system cells have been found in close proximity to HSCs in hematopoietic microenvironments. Their role in influencing HSC fate decisions is also gaining attention.5 RET is well known as a neuronal receptor that receives signals from the glial-derived neurotrophic factor (GDNF) family of ligands and their coreceptors.6 Prior work, using constitutive and conditional knockout mouse models, has shown that RET expression on HSCs is required for their survival and function.7 In contrast, RET signaling stimulation in cultured wild-type mouse HSCs enhances expression of the cell survival factor Bcl2 and results in their ability to generate greater outputs of progeny in vitro and upon transplantation. Despite the HSC-supportive role shown for RET in the mouse context, whether this receptor and its downstream signaling axes may function to promote human HSCs has not yet been evaluated.
Working with primitive cord blood cells, Grey et al identified RET as one of the most differentially active kinases in stem cell–enriched fractions compared with more committed progenitors. They found an approximately fourfold higher frequency of functional HSCs within the subset of REThi as compared with RETlo cells. The effects of RET agonism on human HSCs were explored by providing the primary RET ligand/coreceptor GDNF/GFRα1 to these cells in culture. Using a limiting dilution analysis strategy for retransplanting cells from primary animals reconstituted with GDNF/GFRα1 or control treated HSCs, the authors demonstrated that RET stimulation in vitro resulted in the production of 30 times more long-term HSCs. Interestingly, this expansion was superior to that achieved by a combination SR1/UM171 treatment, which yielded a 15-fold expansion. The authors then monitored the dynamic molecular changes that occurred following RET stimulation. Their analyses indicated that GDNF/GFRα1 propagates downstream signaling that converges on antiapoptosis and anti-inflammation through the activation of the AKT and ERK pathways, interleukin signaling, and the NFκB/p53/BCL2 axis (see figure).
The Grey et al study is notable for its exploration of molecular mechanism through surveying phosphoprotein changes. In particular, the authors use kinome profiling followed at the single-cell level by mass cytometry validations. This is a good strategy to uncover key alterations upon activation of an established receptor tyrosine kinase like RET; however, it is not often implemented in favor of techniques like transcriptomics that have traditionally been more amenable to use for small cell populations. Using this approach, the authors found that GDNF/GFRα1-induced posttranscriptional and posttranslational (phosphoprotein-level) changes were more important than transcriptional changes in modulating central effectors. These results point to the potential for advances in low-input, highly multiplexed protein level measurements to unveil overlooked, yet critical mechanisms of action for other HSC regulators.
In the future, it will be important to pin down the extent to which the in vitro activation of RET signaling is able to expand human HSCs prior to transplant, as well as to determine whether expansions can be further optimized and scaled up. This may include exploring the effects of stimulation over longer culture times (beyond the 7 days tested here), optimizing GDNF/GFRα1 dosing and testing of synergies with other proven approaches for promoting HSCs ex vivo, such as fed batch systems and low-albumin conditions.8,9 In summary, the Grey et al discovery of the importance of RET and its stimulation in expanding human HSCs deepens our understanding of conserved HSC control mechanisms. This study links an environmental factor and its receptor with the intracellular propagation of a concerted network of stem cell–promoting signals. At the same time, this work offers promising insights into new avenues that may advance HSC-based regenerative therapeutics.
Conflict-of-interest disclosure: The author declares no competing financial interests.