Key Points
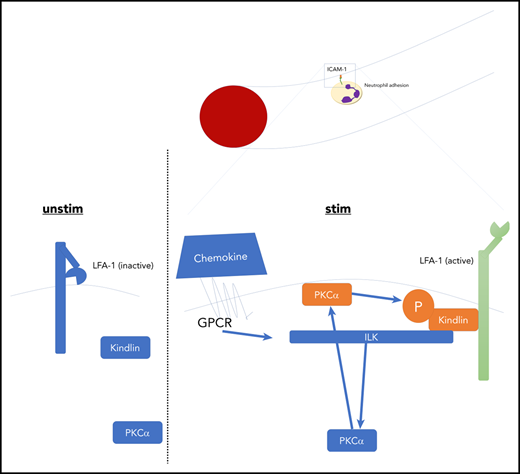
ILK is required for chemokine-induced full activation of LFA-1 by PKC-α–mediated phosphorylation of kindlin-3.
ILK affects chemokine-mediated cell adhesion in the microcirculation and kidney damage after renal ischemia reperfusion injury.
Abstract
Neutrophil adhesion and extravasation into tissue at sites of injury or infection depend on binding of the integrin lymphocyte function–associated antigen 1 (LFA-1) to ICAM-1 expressed on activated endothelial cells. The activation-dependent conformational change of LFA-1 to the high-affinity conformation (H+) requires kindlin-3 binding to the β2-integrin cytoplasmic domain. Here we show that genetic deletion of the known kindlin interactor integrin-linked kinase (ILK) impaired neutrophil adhesion and extravasation in the cremaster muscle and in a clinically relevant model of renal ischemia reperfusion injury. Using in vitro microfluidic adhesion chambers and conformation-specific antibodies, we show that knockdown of ILK in HL-60 cells reduced the conformational change of β2-integrins to the H+ conformation. Mechanistically, we found that ILK was required for protein kinase C (PKC) membrane targeting and chemokine-induced upregulation of its kinase activity. Moreover, PKC-α deficiency also resulted in impaired leukocyte adhesion in bone marrow chimeric mice. Mass spectrometric and western blot analyses revealed stimulation- and ILK-dependent phosphorylation of kindlin-3 upon activation. In summary, our data indicate an important role of ILK in kindlin-3–dependent conformational activation of LFA-1.
Introduction
Organism integrity requires tightly regulated immune defense mechanisms to combat bacterial invasion as well as to prevent autoimmunity. Neutrophils are among the first responders to an inflammatory stimulus and are capable of invading a multitude of organs after a process known as the leukocyte recruitment cascade, involving rolling, adhesion, and extravasation.1 Leukocyte extravasation requires firm binding of leukocyte integrins to counter ligands on endothelial cells. To achieve this, an integrin activation step is necessary, in which the integrin extracellular domain changes its structure from a bent, inactive, closed (E−H−) toward an extended, high-affinity, open conformation (E+H+). This conformational switch exposes the integrin binding site and thus enables adequate binding of the integrin to its ligand.2 It has been suggested that selectin binding leads to the extended closed-headpiece conformation (E+H−), requiring the presence of the integrin adaptor protein talin, whereas chemokine stimulation engages the inside-out response of opening the headpiece to its fully active form, which crucially relies on both talin-1 and kindlin-3.3,4 One major integrin responsible for neutrophil adhesion is the β2-integrin lymphocyte function–associated antigen 1 (LFA-1), capable of interacting with ICAM-1. ILK, a pseudokinase described as a β1-integrin cytoplasmic tail interactor,5 is capable of binding to α-parvin, thus promoting β-integrin engagement and focal adhesion assembly.6 It possesses sites for kindlin binding and was recently implicated in regulation of kindlin-2–dependent integrin functionality.7,8 However, the role of ILK in the regulation of neutrophilic kindlin-3–dependent β2-integrin functionality remains unclear.
Methods
Mice, cell lines, and constructs
Details on animals, cell lines, constructs, ligand binding, clustering, and biochemical assays are included in the supplemental Data, available on the Blood Web site.
In vivo experiments
Two hours after injection of 500 ng of tumor necrosis factor α (TNFα), the cremaster muscle was exteriorized, and images were recorded. Anti–E-selectin (30 µg) antibody was applied at the time of TNFα administration and directly before imaging. For analysis of chemokine-induced arrest, after placement of a carotid artery catheter, 500 ng of rmCXCL1 was administered, and cells were counted under direct observation for 15 minutes.
Statistics
Multigroup (≥3) analyses were performed using 1-way analysis of variance (ANOVA) or respective ANOVA on ranks, followed by post hoc testing. Chemokine-induced arrest analyses over time used 2-way repeated measures ANOVA with Bonferroni post hoc test. Two groups were compared using Student t test or Mann-Whitney rank-sum test as appropriate. P ≤ .05 was considered statistically significant.
Results and discussion
To investigate the role of ILK in neutrophil integrin regulation, we conditionally deleted the ILK gene in myeloid cells (ILKfl/fl/LysMCre). Intravital microscopy of the TNFα-inflamed cremaster muscle revealed reduced leukocyte adhesion and extravasation in ILK-mutant mice compared with ILK wild-type animals in a chemokine-dependent model, whereas leukocyte rolling velocity was not altered11 (Figure 1A-C; supplemental Figure 1A-C). These mice also exhibited reduced leukocyte arrest in response to IV CXCL1 chemokine injection, which is known to be mediated by LFA-112 (Figure 1D), suggesting that ILK is involved in chemokine-induced regulation of β2-integrin activation. From a clinical perspective, control of leukocyte activation and recruitment is crucial to balancing bacterial defense and self-inflicted organ damage (compare sepsis). In a model of ischemia reperfusion–induced acute kidney injury, which depends on integrin activation,13,14 absence of ILK significantly reduced neutrophil recruitment into the kidney, decreased organ damage, and improved kidney function, as shown by lower serum creatinine levels (Figure 1E-G). We then studied integrin activity regulation in ILK knockdown HL-60 cells (supplemental Figure 1D) by reporter flow chamber experiments. These studies revealed that induction of an extended LFA-1 conformation, indicated by KIM127 antibody binding, can occur in the absence of ILK, with other members of the IPP complex still being expressed (supplemental Figure 1E). However, the shift toward the extended open, high-affinity LFA-1 conformation, the induction of which was shown to require kindlin-3 binding to the integrin cytoplasmic domain3 and which is characterized by mAb24 antibody binding, additionally requires the presence of ILK (Figure 1H-I; supplemental Figure 1F-G). Ligand binding assays revealed reduced ICAM-1 but normal fibrinogen binding of ILK-deficient neutrophils, suggesting that LFA-1 is more sensitive to the absence of ILK than fibrinogen binding Mac-1 (Figure 1J-K). This is in line with the observed in vivo phenotype, demonstrating that LFA-1–dependent recruitment steps that are triggered by chemokines were affected, but selectin-mediated adhesion was not (Figure 1L-N; supplemental Figure 2A-B). In contrast to data indicating the necessity of kindlin-3 for both activation and clustering of β3-integrins,15 primary ILK-deficient murine neutrophils showed normal LFA-1 clustering upon chemokine stimulation, suggesting that affinity, but not avidity, is affected by the absence of ILK (supplemental Figure 2C-E). Future studies have to address this controversial point in more detail. Further evaluation of the interplay between ILK and kindlin-3 on LFA-1 activation revealed that knockdown of ILK in green fluorescent protein–tagged kindlin-3–overexpressing HL-60 cells reduced kindlin-3 phosphorylation (Figure 2A), which was not mediated via Akt because its kinase activity remained unchanged (supplemental Figure 3A). As a recent study reported kindlin-3 phosphorylation and its impact on β3-integrin activation in platelets in a PKC-α– or PKC-β–dependent manner,16 we speculated that ILK is involved in PKC-mediated kindlin-3 phosphorylation, which may also influence integrin activity regulation. Indeed, coimmunoprecipitation experiments in native HL-60 cells transfected with green fluorescent protein–tagged kindlin-3 and/or cherry-tagged ILK showed an increased interaction of ILK and PKC, kindlin-3 and PKC, and ILK and kindlin-3 following chemokine stimulation (supplemental Figure 3B-D). Administration of a PKC-α inhibitor in wild-type animals significantly reduced chemokine-mediated leukocyte arrest in vivo (Figure 2B), which is consistent with a recently published constitutively active PKC mutant showing increased LFA-1 binding to ICAM-1.17 Inhibiting PKC-α in ILK-deficient mice had no further effect on chemokine-induced leukocyte arrest in vivo (Figure 2B). To directly test the role of PKC-α in neutrophil adhesion, we generated chimeric mice with PKC-α–deficient bone marrow, which exhibited a significant reduction in the number of arrested neutrophils in vivo after chemokine administration (Figure 2C). Confocal microscopy and colocalization analyses of human neutrophils showed membrane targeting of kindlin-3 upon IL-8 stimulation (supplemental Figure 3E) and chemokine (IL-8)–dependent colocalization of PKC-α and kindlin-3 (Figure 2D). Interestingly, we found that PKC membrane translocation and kinase activity were both reduced in the absence of ILK, which may explain the reduced kindlin-3 phosphorylation in ILK knockdown cells (Figure 2E-F). To identify putative chemokine-induced phosphorylation of kindlin-3, we stimulated HL-60 cells with IL-8 and analyzed cell lysates by mass spectrometry. Several large-scale phosphoproteomics LC-MSMS experiments identified a phosphorylated serine at position 484 within the tryptic peptide sequence TGpSGGPGNH PHGPDASAEG LNPYGLVAPR of human kindlin-3 protein (supplemental Figure 4); however, phosphorylation of threonine on position 482 could not be ruled out by these measurements (supplemental Figure 5). Neural network–based kinase prediction (NetPhos 3.1)18,19 led us to hypothesize that serine at position 485 or serine at position 481 in murine kindlin-3 might be phosphorylated in a PKC-dependent manner (no serine is present at position 484 in mice; supplemental Figure 6). Transfection of a nonphosphorylatable mutant S485A in kindlin-3–deficient neutrophils and subsequent analysis of LFA-1 activity revealed a reduction in ICAM-1 binding corroborating that phosphorylation of kindlin-3 at S485 but not at S481 contributes to integrin activation (Figure 2G). This is in line with recent work in platelets and HEL cells demonstrating PKC-mediated S484 phosphorylation of kindlin-3 during β3-integrin activation, in which a T482/S484 kindlin-3 mutant failed to adequately activate the α2bβ3-integrin.16,20
ILK affects leukocyte adhesion by control of the LFA-1 high-affinity conformation. (A-C) Animals were injected intrascrotally with TNFα and anti–E-selectin IV 2 hours before imaging. Upon imaging, animals were reinjected with anti–E-selectin, and analyses were performed for adherent leukocytes (A), rolling velocity (B), and transmigration (C) (n = 4). (D) For analysis of chemokine-induced arrest, the cremaster muscle was prepared, and CXCL-1 was administered via a carotid artery catheter. Subsequent cellular arrest was assessed by observation through an intravital microscope (n = 6). (E-G) Control and knockout (KO) animals were subjected to induction of acute kidney damage, and neutrophil infiltration per kidney, increase in serum creatinine, and histological changes were assessed (n = 4-6, scale bar in (F) indicates 100 µm, hematoxylin and eosin stain). (H-I) Conformation-specific antibodies detecting the extended (KIM127 [H]) or high-affinity (mAb24 [I]) conformation of integrins were coated in a microfluidic chamber setup. Arrested cells per field of view are depicted in the graph (n = 4-6). (J-K) Binding of the β2-integrin binding partners fibrinogen (Mac-1; n = 3) (J) and ICAM-1 (LFA-1; n = 4) (K) was assessed by flow cytometry. Cells were stimulated with CXCL-1, and binding response was analyzed by fluorescent coupled ligand binding. (L) Focusing on selectin-mediated leukocyte recruitment revealed that there was no difference between wild-type (WT) and ILK-deficient mice regarding the number of adherent cells. For this purpose, the in vivo cremaster muscle model was applied, in which animals received an intrascrotal injection of TNFα 2 hours before imaging. To block G protein–coupled receptor (GPCR)–induced chemokine signaling, pertussis toxin (4 µg) was injected IV at the time of TNFα administration and directly before preparation of the cremaster muscle. These data corroborate that ILK in our model exclusively affected the chemokine-mediated recruitment of cells (n = 3). (M) In accordance, an ex vivo autoperfused micro flow chamber setup showed no difference in rolling velocities of leukocytes on E-selectin alone or E-selectin and ICAM-1 (n = 3-5). (N) Blocking of both GPCR and selectin signaling led to comparable low numbers of adherent cells between the 2 groups (n = 3). Data are presented as mean ± standard error of the mean. *P < .05, ***P < .001. FOV, field of view; IgG, immunoglobulin G; IRI, ischemia reperfusion injury; KD, knockdown; MFI, mean fluorescence intensity; n.s., not significant; PMN, polymorphonuclear neutrophil; SCR, scrambled; stim, stimulated; unstim, unstimulated.
ILK affects leukocyte adhesion by control of the LFA-1 high-affinity conformation. (A-C) Animals were injected intrascrotally with TNFα and anti–E-selectin IV 2 hours before imaging. Upon imaging, animals were reinjected with anti–E-selectin, and analyses were performed for adherent leukocytes (A), rolling velocity (B), and transmigration (C) (n = 4). (D) For analysis of chemokine-induced arrest, the cremaster muscle was prepared, and CXCL-1 was administered via a carotid artery catheter. Subsequent cellular arrest was assessed by observation through an intravital microscope (n = 6). (E-G) Control and knockout (KO) animals were subjected to induction of acute kidney damage, and neutrophil infiltration per kidney, increase in serum creatinine, and histological changes were assessed (n = 4-6, scale bar in (F) indicates 100 µm, hematoxylin and eosin stain). (H-I) Conformation-specific antibodies detecting the extended (KIM127 [H]) or high-affinity (mAb24 [I]) conformation of integrins were coated in a microfluidic chamber setup. Arrested cells per field of view are depicted in the graph (n = 4-6). (J-K) Binding of the β2-integrin binding partners fibrinogen (Mac-1; n = 3) (J) and ICAM-1 (LFA-1; n = 4) (K) was assessed by flow cytometry. Cells were stimulated with CXCL-1, and binding response was analyzed by fluorescent coupled ligand binding. (L) Focusing on selectin-mediated leukocyte recruitment revealed that there was no difference between wild-type (WT) and ILK-deficient mice regarding the number of adherent cells. For this purpose, the in vivo cremaster muscle model was applied, in which animals received an intrascrotal injection of TNFα 2 hours before imaging. To block G protein–coupled receptor (GPCR)–induced chemokine signaling, pertussis toxin (4 µg) was injected IV at the time of TNFα administration and directly before preparation of the cremaster muscle. These data corroborate that ILK in our model exclusively affected the chemokine-mediated recruitment of cells (n = 3). (M) In accordance, an ex vivo autoperfused micro flow chamber setup showed no difference in rolling velocities of leukocytes on E-selectin alone or E-selectin and ICAM-1 (n = 3-5). (N) Blocking of both GPCR and selectin signaling led to comparable low numbers of adherent cells between the 2 groups (n = 3). Data are presented as mean ± standard error of the mean. *P < .05, ***P < .001. FOV, field of view; IgG, immunoglobulin G; IRI, ischemia reperfusion injury; KD, knockdown; MFI, mean fluorescence intensity; n.s., not significant; PMN, polymorphonuclear neutrophil; SCR, scrambled; stim, stimulated; unstim, unstimulated.
ILK affects protein kinase C α (PKC-α)–mediated phosphorylation of kindlin-3. (A) HL-60 cells were transfected with N-terminally green fluorescent protein (GFP)–tagged kindlin-3 and stimulated (stim) with interleukin-8 (IL-8) for indicated durations (100 nM), and GFP-trap–based immunoprecipitation was performed. Subsequent blotting with a serine phosphorylation–specific antibody revealed reduced phosphorylation of GFP-tagged kindlin-3 in ILK-deficient cells. Stimulation times (no stimulation [unstim ctrl], 30 seconds, and 3 minutes) are depicted above the representative image (n = 3-4). Analyses of chemokine (CXCL1)–induced arrest in mice treated with a PKC-α inhibitor or vehicle control (B) and of chimeric mice with PKC-α–deficient bone marrow (C) revealed reduced chemokine (CXCL1)–induced integrin-mediated leukocyte arrest in vivo (n = 3-5). For this purpose, mice were anesthetized, a carotid artery catheter was placed, and the mouse cremaster muscle was prepared. Upon direct observation via intravital microscopy, the chemokine CXCL1 was injected, and the arrest of leukocytes was assessed. (D) Analysis of isolated human neutrophils, stimulated with IL-8, showed a chemokine-dependent colocalization (colocalization channel in gray; left) of PKC-α (blue) and kindlin-3 (red) (n = 4). Images were recorded on a Zeiss LSM 700 confocal microscope equipped with a 63× oil immersion objective. (E) IL-8 chemokine stimulation of HL-60 cells led to membrane localization of PKC in control cells, whereas this translocation was absent in ILK-deficient cells (n = 3-5). (F) Furthermore, PKC activity as assessed by radioactivity measurement of substrate turnover revealed decreased PKC activity in ILK-deficient cells in control conditions vs stimulation for 3 minutes with IL-8 (n = 6). (G) Bone marrow from kindlin-3–deficient animals was transfected with different kindlin-3 mutants and subsequently differentiated as previously reported.23 Cells were stimulated with CXCL1 and used for ICAM-1 binding assay. Lines above graph indicate significance among groups (n = 2-3 independent transfections; n ≥ 3 experiments). *P ≤ .05, **P < .01. FI, fold increase; IP, immunoprecipitation; KD, knockdown; KO, knockout; MFI, mean fluorescence intensity; n.s., not significant; SCR, scrambled.
ILK affects protein kinase C α (PKC-α)–mediated phosphorylation of kindlin-3. (A) HL-60 cells were transfected with N-terminally green fluorescent protein (GFP)–tagged kindlin-3 and stimulated (stim) with interleukin-8 (IL-8) for indicated durations (100 nM), and GFP-trap–based immunoprecipitation was performed. Subsequent blotting with a serine phosphorylation–specific antibody revealed reduced phosphorylation of GFP-tagged kindlin-3 in ILK-deficient cells. Stimulation times (no stimulation [unstim ctrl], 30 seconds, and 3 minutes) are depicted above the representative image (n = 3-4). Analyses of chemokine (CXCL1)–induced arrest in mice treated with a PKC-α inhibitor or vehicle control (B) and of chimeric mice with PKC-α–deficient bone marrow (C) revealed reduced chemokine (CXCL1)–induced integrin-mediated leukocyte arrest in vivo (n = 3-5). For this purpose, mice were anesthetized, a carotid artery catheter was placed, and the mouse cremaster muscle was prepared. Upon direct observation via intravital microscopy, the chemokine CXCL1 was injected, and the arrest of leukocytes was assessed. (D) Analysis of isolated human neutrophils, stimulated with IL-8, showed a chemokine-dependent colocalization (colocalization channel in gray; left) of PKC-α (blue) and kindlin-3 (red) (n = 4). Images were recorded on a Zeiss LSM 700 confocal microscope equipped with a 63× oil immersion objective. (E) IL-8 chemokine stimulation of HL-60 cells led to membrane localization of PKC in control cells, whereas this translocation was absent in ILK-deficient cells (n = 3-5). (F) Furthermore, PKC activity as assessed by radioactivity measurement of substrate turnover revealed decreased PKC activity in ILK-deficient cells in control conditions vs stimulation for 3 minutes with IL-8 (n = 6). (G) Bone marrow from kindlin-3–deficient animals was transfected with different kindlin-3 mutants and subsequently differentiated as previously reported.23 Cells were stimulated with CXCL1 and used for ICAM-1 binding assay. Lines above graph indicate significance among groups (n = 2-3 independent transfections; n ≥ 3 experiments). *P ≤ .05, **P < .01. FI, fold increase; IP, immunoprecipitation; KD, knockdown; KO, knockout; MFI, mean fluorescence intensity; n.s., not significant; SCR, scrambled.
Taken together, these results exemplify the relevance of ILK-dependent phosphorylation of kindlin-3 by PKC-α for full activation of the β2-integrin LFA-1 triggered by chemokines and its consequences for leukocyte adhesion and recruitment. However, it seems that this signaling event is not the exclusive regulatory key for full integrin activation but rather a delicate further activational switch, because integrin activation in ILK-deficient cells was not fully blunted, as was observed in kindlin-3 knockout cells. Further research is therefore needed to dissect the additional steps underlying the successful extension and/or opening of the integrin headpiece in more detail21 and assess similar signaling processes involved in Mac-1 activation.22
Original data are available upon reasonable request to the corresponding author.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Katharina Körner, Birgit Schulte, and Anja Standke for expert technical assistance and Reinhard Fässler for providing the ILK-deficient animals and expert advice and discussion of the topic.
This work was supported by German Research Foundation grants ZA428/12-1, SFB1009/A05, INST 211/604-2, KFO 342/1, and ZA428/18-1 (A.Z.) and RO4537/4-1 and RO4537/5-1 (J.R.) and by Interdisciplinary Center for Clinical Research grants IZKF Za2/001/18 (A.Z.) and IZKF SEED12/18 (A.M.).
Authorship
Contribution: G.G., A.M., H.C.A.D., J.R., S.M., K.T., N.L., B.P., H.B., W.G., C.L., P.W.K., and M.M.z.B. performed experiments. G.G., A.M., H.C.A.D., J.R., S.M., K.T., N.L., H.B., C.L., D.V., and A.Z. analyzed and interpreted data. A.M., G.G., and A.Z. wrote the manuscript. P.W.K., H.H., B.H., M.M., and D.V. contributed to writing the manuscript and discussed data. A.Z. conceived of and supervised the study.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Alexander Zarbock, University of Muenster, Department of Anesthesiology, Intensive Care Medicine and Pain Therapy, Albert-Schweitzer-Campus 1, Building A1, 48149 Muenster, Germany; e-mail: zarbock@uni-muenster.de.
REFERENCES
Author notes
A.M. and G.G. contributed equally to this work.

![ILK affects leukocyte adhesion by control of the LFA-1 high-affinity conformation. (A-C) Animals were injected intrascrotally with TNFα and anti–E-selectin IV 2 hours before imaging. Upon imaging, animals were reinjected with anti–E-selectin, and analyses were performed for adherent leukocytes (A), rolling velocity (B), and transmigration (C) (n = 4). (D) For analysis of chemokine-induced arrest, the cremaster muscle was prepared, and CXCL-1 was administered via a carotid artery catheter. Subsequent cellular arrest was assessed by observation through an intravital microscope (n = 6). (E-G) Control and knockout (KO) animals were subjected to induction of acute kidney damage, and neutrophil infiltration per kidney, increase in serum creatinine, and histological changes were assessed (n = 4-6, scale bar in (F) indicates 100 µm, hematoxylin and eosin stain). (H-I) Conformation-specific antibodies detecting the extended (KIM127 [H]) or high-affinity (mAb24 [I]) conformation of integrins were coated in a microfluidic chamber setup. Arrested cells per field of view are depicted in the graph (n = 4-6). (J-K) Binding of the β2-integrin binding partners fibrinogen (Mac-1; n = 3) (J) and ICAM-1 (LFA-1; n = 4) (K) was assessed by flow cytometry. Cells were stimulated with CXCL-1, and binding response was analyzed by fluorescent coupled ligand binding. (L) Focusing on selectin-mediated leukocyte recruitment revealed that there was no difference between wild-type (WT) and ILK-deficient mice regarding the number of adherent cells. For this purpose, the in vivo cremaster muscle model was applied, in which animals received an intrascrotal injection of TNFα 2 hours before imaging. To block G protein–coupled receptor (GPCR)–induced chemokine signaling, pertussis toxin (4 µg) was injected IV at the time of TNFα administration and directly before preparation of the cremaster muscle. These data corroborate that ILK in our model exclusively affected the chemokine-mediated recruitment of cells (n = 3). (M) In accordance, an ex vivo autoperfused micro flow chamber setup showed no difference in rolling velocities of leukocytes on E-selectin alone or E-selectin and ICAM-1 (n = 3-5). (N) Blocking of both GPCR and selectin signaling led to comparable low numbers of adherent cells between the 2 groups (n = 3). Data are presented as mean ± standard error of the mean. *P < .05, ***P < .001. FOV, field of view; IgG, immunoglobulin G; IRI, ischemia reperfusion injury; KD, knockdown; MFI, mean fluorescence intensity; n.s., not significant; PMN, polymorphonuclear neutrophil; SCR, scrambled; stim, stimulated; unstim, unstimulated.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/136/19/10.1182_blood.2020004948/2/m_bloodbld2020004948f1.png?Expires=1767791146&Signature=Ki4tyM70tSjsH1BO7kbKnQrdttc9SGyph1PWnh7rDUoStl7WMslksvS8HjY4ntV2Y0~NlX--mat10U1VTRY~LOayiZDblviBnMdQz5sp0F2qdxaPgzKwqDwyoNcz80VyRoQqyAHghiQt4dhB6OZroJqUgBDqg5W1etMPJKp58yW9LwkLLlqkbVEH01ujCktJMOPtKSwplvuz3o-Aj9lti6YwCGIXIDgJcFYbWzzLpOakkhjjR1Awntb9HPKLB3uZvi~d0uFwS6X1MBYffFe182mTDeY9-JAiG~t8jsgnx7umwGCIurYZPgGoYpoX2UW9JfEO3vQumKxDcBcblOCjVA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![ILK affects protein kinase C α (PKC-α)–mediated phosphorylation of kindlin-3. (A) HL-60 cells were transfected with N-terminally green fluorescent protein (GFP)–tagged kindlin-3 and stimulated (stim) with interleukin-8 (IL-8) for indicated durations (100 nM), and GFP-trap–based immunoprecipitation was performed. Subsequent blotting with a serine phosphorylation–specific antibody revealed reduced phosphorylation of GFP-tagged kindlin-3 in ILK-deficient cells. Stimulation times (no stimulation [unstim ctrl], 30 seconds, and 3 minutes) are depicted above the representative image (n = 3-4). Analyses of chemokine (CXCL1)–induced arrest in mice treated with a PKC-α inhibitor or vehicle control (B) and of chimeric mice with PKC-α–deficient bone marrow (C) revealed reduced chemokine (CXCL1)–induced integrin-mediated leukocyte arrest in vivo (n = 3-5). For this purpose, mice were anesthetized, a carotid artery catheter was placed, and the mouse cremaster muscle was prepared. Upon direct observation via intravital microscopy, the chemokine CXCL1 was injected, and the arrest of leukocytes was assessed. (D) Analysis of isolated human neutrophils, stimulated with IL-8, showed a chemokine-dependent colocalization (colocalization channel in gray; left) of PKC-α (blue) and kindlin-3 (red) (n = 4). Images were recorded on a Zeiss LSM 700 confocal microscope equipped with a 63× oil immersion objective. (E) IL-8 chemokine stimulation of HL-60 cells led to membrane localization of PKC in control cells, whereas this translocation was absent in ILK-deficient cells (n = 3-5). (F) Furthermore, PKC activity as assessed by radioactivity measurement of substrate turnover revealed decreased PKC activity in ILK-deficient cells in control conditions vs stimulation for 3 minutes with IL-8 (n = 6). (G) Bone marrow from kindlin-3–deficient animals was transfected with different kindlin-3 mutants and subsequently differentiated as previously reported.23 Cells were stimulated with CXCL1 and used for ICAM-1 binding assay. Lines above graph indicate significance among groups (n = 2-3 independent transfections; n ≥ 3 experiments). *P ≤ .05, **P < .01. FI, fold increase; IP, immunoprecipitation; KD, knockdown; KO, knockout; MFI, mean fluorescence intensity; n.s., not significant; SCR, scrambled.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/136/19/10.1182_blood.2020004948/2/m_bloodbld2020004948f2-1.png?Expires=1767791146&Signature=WpDxtlbXMqfriRFHrOvColRil875qylgwDcEeK3sW2taxAPFEZfsv2n9baEdv2Plt0fCPucabchxL-3qRyXT6piuln-lHzKZxp3Mm-SjLedsoXHqHBGBZxL8jej87DLHb5b1LtvHjtHwPAyxytgKPJffuYb7bSn~v9WudcmKW7he9GJgg-hrTZu8EFacpOsMfZd~0jiHeXziilg~tKqoB77bn~b7WEWkqphhJuuzNc0if5WcS~twW1~IekUjXjudUGnz8LdqcuU~8O1m9~-A5N41hjfBannCFDAIBFPHOYBgaMiqoxJJX5qAMfqoLBTQKeaVwoAMYDiMLveWfyJBzA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![ILK affects protein kinase C α (PKC-α)–mediated phosphorylation of kindlin-3. (A) HL-60 cells were transfected with N-terminally green fluorescent protein (GFP)–tagged kindlin-3 and stimulated (stim) with interleukin-8 (IL-8) for indicated durations (100 nM), and GFP-trap–based immunoprecipitation was performed. Subsequent blotting with a serine phosphorylation–specific antibody revealed reduced phosphorylation of GFP-tagged kindlin-3 in ILK-deficient cells. Stimulation times (no stimulation [unstim ctrl], 30 seconds, and 3 minutes) are depicted above the representative image (n = 3-4). Analyses of chemokine (CXCL1)–induced arrest in mice treated with a PKC-α inhibitor or vehicle control (B) and of chimeric mice with PKC-α–deficient bone marrow (C) revealed reduced chemokine (CXCL1)–induced integrin-mediated leukocyte arrest in vivo (n = 3-5). For this purpose, mice were anesthetized, a carotid artery catheter was placed, and the mouse cremaster muscle was prepared. Upon direct observation via intravital microscopy, the chemokine CXCL1 was injected, and the arrest of leukocytes was assessed. (D) Analysis of isolated human neutrophils, stimulated with IL-8, showed a chemokine-dependent colocalization (colocalization channel in gray; left) of PKC-α (blue) and kindlin-3 (red) (n = 4). Images were recorded on a Zeiss LSM 700 confocal microscope equipped with a 63× oil immersion objective. (E) IL-8 chemokine stimulation of HL-60 cells led to membrane localization of PKC in control cells, whereas this translocation was absent in ILK-deficient cells (n = 3-5). (F) Furthermore, PKC activity as assessed by radioactivity measurement of substrate turnover revealed decreased PKC activity in ILK-deficient cells in control conditions vs stimulation for 3 minutes with IL-8 (n = 6). (G) Bone marrow from kindlin-3–deficient animals was transfected with different kindlin-3 mutants and subsequently differentiated as previously reported.23 Cells were stimulated with CXCL1 and used for ICAM-1 binding assay. Lines above graph indicate significance among groups (n = 2-3 independent transfections; n ≥ 3 experiments). *P ≤ .05, **P < .01. FI, fold increase; IP, immunoprecipitation; KD, knockdown; KO, knockout; MFI, mean fluorescence intensity; n.s., not significant; SCR, scrambled.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/136/19/10.1182_blood.2020004948/2/m_bloodbld2020004948f2-2.png?Expires=1767791146&Signature=dF~BBzSW54Z3eQZ8oWgJmLdJvA~UhS5Vl2~49BmwHemp39DNC1gzSK6q8V8DUsnVMn-ip7rp263hbfxfy8RNaJ~zA~2l~CzkqVc2zxAcuUh8Ba24hA6o4kxb60-H5W4O7Kne4KsLNSGJs1IcR3hcwhR50VR5xICKZaXTh8EmJsaB6zxpKOlE-fPcYScGnJZXDJM0FAbAWCxIZ1Q9dYaQcoeosYy7O2eej-60yICayRgPOxSlYnLkYf~3EAzMCfJnjmgEOCeePFQNYT4FFrKfTljMMlaN4~moHngAup7JztylpfdZqVocppVmLSdiH7Jb0TK21-WRMZiMpFHDD7L9xw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal