In this issue of Blood, Sun and colleagues present results from a randomized, phase 2 study of acalabrutinib at either 100 mg twice daily or 200 mg daily in patients with treatment-naive or relapsed/refractory chronic lymphocytic leukemia (CLL).1 As part of the study, they undertook a rigorous analysis of Bruton tyrosine kinase (BTK) occupancy and the resulting biologic consequences in different tissue compartments. They established that twice-daily dosing achieved higher BTK occupancy and resultant downstream pathway inhibition in lymph nodes than once-daily dosing and established the rate of BTK resynthesis in CLL cells.
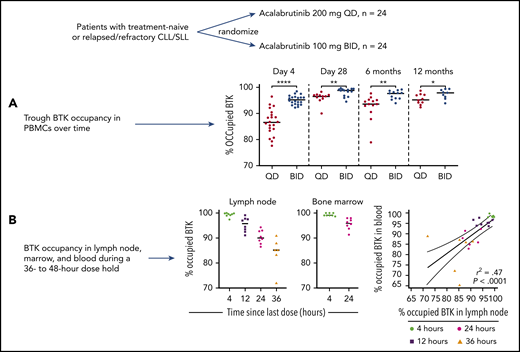
Patients with high-risk treatment-naive or relapsed/refractory CLL/small lymphocytic lymphoma were randomized to 200 mg daily (QD) or 100 mg twice daily (BID) of acalabrutinib. (A) Trough BTK occupancy was serially measured (at day 4 and 1, 6, and 12 months) in PBMCs, showing higher occupancy in the twice-daily dosing cohort at all time points and increased BTK occupancy over time. (B) BTK occupancy in PBMC, bone marrow, and lymph nodes was measured during a 36- to 48-hour dose hold, allowing calculation of BTK resynthesis rates and demonstrating close correlation between BTK occupancy in PBMCs and in tissue compartments. *P ≤ .05; **P ≤ .01; ****P ≤ .0001. The figure has been adapted from Figures 4 and 5 in the article by Sun et al that begins on page 93. Professional illustration by Brian Cannon.
Patients with high-risk treatment-naive or relapsed/refractory CLL/small lymphocytic lymphoma were randomized to 200 mg daily (QD) or 100 mg twice daily (BID) of acalabrutinib. (A) Trough BTK occupancy was serially measured (at day 4 and 1, 6, and 12 months) in PBMCs, showing higher occupancy in the twice-daily dosing cohort at all time points and increased BTK occupancy over time. (B) BTK occupancy in PBMC, bone marrow, and lymph nodes was measured during a 36- to 48-hour dose hold, allowing calculation of BTK resynthesis rates and demonstrating close correlation between BTK occupancy in PBMCs and in tissue compartments. *P ≤ .05; **P ≤ .01; ****P ≤ .0001. The figure has been adapted from Figures 4 and 5 in the article by Sun et al that begins on page 93. Professional illustration by Brian Cannon.
BTK is part of the B-cell receptor (BCR) signaling pathway that is important in CLL pathogenesis.2,3 BTK inhibitors are highly effective treatments for treatment-naive and relapsed/refractory CLL.4 The BTK inhibitors currently approved by the Food and Drug Administration (FDA) for treatment of B-cell malignancies all irreversibly inhibit BTK by binding to the C481 residue of BTK. Although all 3 FDA-approved BTK inhibitors, ibrutinib, acalabrutinib, and zanubrutinib, have short plasma half-lives (2 to 3 hours, 1 hour, and 4 hours, respectively), they have sustained biologic activity due to covalent binding to BTK.5-7
The phase 1 studies of covalent BTK inhibitors did not identify maximal tolerated doses.5-7 Instead, the doses for subsequent studies were selected on the basis of an optimal biological effect, assessed by BTK occupancy. The phase 1 study of acalabrutinib demonstrated that all doses tested (100 mg to 400 mg daily and 100 mg twice daily) achieved excellent BTK inhibition 4 hours postdosing, but the 100-mg twice-daily dose demonstrated optimal inhibition 24 hours after dosing.6 One potential limitation to the analyses performed in the phase 1 studies of ibrutinib and acalabrutinib was that BTK occupancy was measured in peripheral blood mononuclear cells (PBMCs) rather than in tissue compartments, due to the comparative ease of collecting these specimens. However, most BCR activation and resultant cell proliferation occur within lymph nodes.8 Although treatment with BTK inhibition leads to a transient redistribution lymphocytosis, BTK occupancy measured in PBMCs could theoretically overestimate the true degree of inhibition seen in CLL cells that remain in lymph nodes. Given clinical dosing strategies for ibrutinib and acalabrutinib have been based on BTK occupancy data measured in PBMCs, it is important to demonstrate that BTK occupancy in lymph node–resident CLL cells can be precisely inferred from the PBMC data. Certainly, in the phase 1 study of zanubrutinib, BTK occupancy in lymph nodes was more variable than PBMC data, especially during once-daily dosing.
As expected, clinical survival outcomes in the current study were favorable, and adverse events occurred at a similar rate as those described in other, larger studies. Of more value than the clinical results are the novel insights provided into BTK occupancy in lymph nodes and the resulting biological effects seen in the different dosing cohorts.
The pharmacokinetic properties of acalabrutinib, with a half-life of ∼1 hour and irreversible inhibition of BTK, mean that decline in BTK occupancy from drug peak to trough represents resynthesis of BTK within CLL cells, occurring at a time when there is no significant quantity of free drug remaining in plasma. Sun et al showed significantly greater BTK occupancy at drug trough in the patients receiving 100 mg twice daily compared with those receiving 200 mg daily, after 1, 6, and 12 months of therapy, supporting the 100-mg twice-daily dosing schedule for acalabrutinib used in routine clinical practice. Trough BTK occupancy increased with more prolonged treatment, commensurate with the previous observation that total BTK declines over time during continuous BTK inhibitor therapy (see figure panel A).9
In addition to these analyses, Sun et al measured BTK occupancy in paired PBMC and lymph node or bone marrow biopsy specimens, during a planned 36- to 48-hour dosing interruption from day 3 to 5. This allowed them to determine trough BTK occupancy in different tissue compartments and to directly calculate the rate of BTK resynthesis. BTK occupancy was higher at drug trough in lymph nodes within the 100-mg twice-daily than the 200-mg daily cohort (95.8% vs 90.1%). Resynthesis rates for BTK were similar in PBMC and in lymph nodes (14.5% vs 11.2% per day), with a tight correlation seen between BTK occupancy in PBMCs and bone marrow or lymph nodes at all time points (see figure panel B). This is critical information for any future studies of BTK occupancy during acalabrutinib therapy, as it demonstrates that testing of BTK occupancy in PBMC samples, which are far more readily obtained, can be reasonably used to infer BTK occupancy in lymph nodes.
Importantly, this study did not just perform BTK occupancy analysis. Transcriptomic analysis using RNA sequencing from purified circulating tumor cells and from whole lymph nodes revealed suppression of gene signatures related to BCR, NF-κB, cytokine signaling, and cellular metabolism. These pathways were more profoundly impacted by twice-daily than daily dosing, and these differences became more pronounced over time, indicating the biological importance of different levels of BTK occupancy and supporting twice-daily dosing.
Taken together, these data are supportive of the current 100-mg twice-daily dosing of acalabrutinib in CLL. However, several questions remain. First, although it appears from the correlative data that the 100-mg twice-daily dosing provides optimal target coverage and biological effect, the study was not powered to detect differences in clinical outcome between the 100-mg twice-daily group and the 200-mg daily group, so the clinical importance of these findings remains uncertain. Second, although 100-mg twice-daily dosing appeared to provide optimal target coverage, it is not clear whether the 100-mg dose is necessary to achieve this, or whether twice-daily dosing using lower doses of acalabrutinib could provide similar target coverage. Third, the study demonstrated that BTK occupancy at drug trough increased over time; consequently, could lower doses be used at later time points? A pilot study demonstrated that sequentially reducing ibrutinib dose from 420 mg/d to 140 mg/d over 3 months achieved >95% BTK occupancy in PBMCs at all dose levels.10 A confirmatory randomized study is planned. Exploration of reduced doses of BTK inhibitors is attractive, as lower doses could attenuate costs, and potentially, toxicity. However, until clinical data are available demonstrating equivalent efficacy of lower doses, BTK inhibitors should be dosed at FDA-approved doses, unless toxicity mandates dose reduction.
Conflict-of-interest disclosure: The author has received speaking fees from Janssen Oncology Australia and has served on advisory boards for Pharmacyclics, AbbVie, Genentech, and Gilead Sciences; The University of Texas MD Anderson Cancer Center has received funds from AstraZeneca, Pharmacyclics, AbbVie, and Genentech for the conduct of clinical research.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal