TO THE EDITOR:
The discovery of PIEZO proteins has enabled better understanding of how cells respond to mechanical force.1-3 PIEZOs assemble to form ion channels that link force to cell behavior via transmembrane cation flux.1,2,4 Shortly after the initial PIEZO discoveries, associations of PIEZO1 mutations with xerocytosis/dehydrated hereditary stomatocytosis (DHS)4-15 and links to malarial resistance were suggested.16 PIEZO1 channels are now an established feature of red blood cell (RBC) biology where they regulate intracellular Ca2+ and cell hydration in coordination with other mechanisms such as the Gardos channel.17-20 However, studies of PIEZO1 reconstituted in cell lines have suggested that the channels activate within milliseconds and then inactivate (desensitize) completely within a few hundred milliseconds when rapidly stimulated by increases in membrane tension caused by pulling on the membrane or cell deformation via a probe.1,4,7,9,18 DHS mutations have been found to slow the fast inactivation process, which represents gain of function.7-9,18 It is, however, unclear how such rapid events are relevant to RBC physiology. Moreover, although the available data are limited because of the low prevalence of DHS, studies of RBCs from patients suggest sustained channel activity unlike that reported for PIEZO1 in cell lines.6,10,12,15 Therefore, we sought additional understanding through electrophysiological analysis of murine RBCs. To mechanically stimulate the channels, we applied shear stress, a frictional force created physiologically by blood flow. To understand the impact of DHS mutation, we generated the murine equivalent of one of the first-identified DHS mutations, M2225R.4,5,8,9
In mouse PIEZO1, the equivalent of M2225R is M2241R. Molecular modeling and pharmacological analysis of overexpressed channels suggested suitability of mouse PIEZO1 as a model for human PIEZO1 (supplemental Results; supplemental Figures 1 and 2, available on the Blood Web site). Furthermore, 8-week-old mice homozygous for M2241R (PIEZO1M-R/M-R; supplemental Results; supplemental Figures 3 and 4) displayed features consistent with DHS that included stomatocytosis (supplemental Figure 5); decreased osmotic fragility (supplemental Figure 6); decreased hemoglobin, hematocrit, and RBC count (supplemental Table 1); and increased RBC and hemoglobin concentration distribution widths and percentage of reticulocytes (supplemental Table 1). There were no significant changes in spleen weight (supplemental Figure 7) or liver function test results (supplemental Table 2). There were potential changes in plasma iron and total iron binding capacity that did not reach statistical significance, but transferrin showed a small significant increase (supplemental Table 2). PIEZO1M-R/M-R mice were born at slightly lower frequency than wild-type (WT) mice, suggesting a potential deleterious effect, but the adult PIEZO1M-R/M-R mice appeared normal, and body weight gain was not different from that in WT mice (supplemental Figure 8). However, increased spleen weight in the PIEZO1M-R/M-R mice at 22 weeks of age suggests the potential for age-related changes in phenotype (supplemental Figure 7). All remaining experiments focused on mice at 8 weeks of age. Heterozygous mice (PIEZO1WT/M-R) showed a decrease in osmotic fragility, but less than for the PIEZO1M-R/M-R mice, which suggests an intermediate phenotype (supplemental Figure 6). The PIEZO1WT/M-R mice were born at the expected Mendelian ratio (supplemental Figure 8).
To understand the effect of M2241R on channel properties, patch-clamp electrophysiology was applied to RBCs freshly isolated from mice. For physiological relevance, we first used the perforated-patch whole-cell configuration to achieve membrane potential measurements. Resting membrane potential data are provided in the supplemental Information. For most experiments, constant current was injected to hyperpolarize RBCs to −80 mV and thereby maximize visibility of PIEZO1-related responses to fluid flow of 20 μL·s−1, a rate that occurs in mice.21 There was a small depolarization in response to the fluid flow in WT RBCs, consistent with the opening of PIEZO1 channels (Figure 1A; mean data in Figure 1D). The depolarization was sustained for at least 20 seconds. After flow ceased, the membrane potential returned to its initial value (Figure 1A,D). Depolarization tended to be larger in PIEZO1WT/M-R and was larger (P < .05) in PIEZO1M-R/M-R RBCs, but the most striking difference was failure to recover after flow ceased (Figure 1B-C; mean data in Figure 1D). To further investigate, we switched to voltage-clamp mode. In WT RBCs, fluid flow caused inward current as expected, which then decayed slowly after about 10 seconds (Figure 1F; mean data in Figure 1I). In PIEZO1WT/M-R and PIEZO1M-R/M-R RBCs, the initial response was similar to that in WT, except the slow inactivation was mostly absent (Figure 1G,H). Most striking, there was failure of current to recover after cessation of fluid flow (Figure 1G-H; mean data in Figure 1I). GsMTx4, an inhibitor of PIEZO1 channel activity,22 abolished all depolarizing and inward current activities (Figure 1E,J; supplemental Figures 9 and 10). An intriguing finding was that washout of GsMTx4 from mutant RBCs revealed recovery to activity that was more like the activity of WT RBCs than that of mutant RBCs in the absence of toxin, suggesting the potential to correct mutant channel behavior (supplemental Figures 9 and 10).
Slow kinetics of RBC PIEZO1 mice and slow recovery in PIEZO1WT/M-R and PIEZO1M-R/M-R mice. (A-C) Membrane potential (Vm) recordings obtained using the perforated-patch, whole-cell mode applied to freshly isolated RBCs from WT (A), PIEZO1WT/M-R (B), and PIEZO1M-R/M-R (C) mice. RBCs were exposed to 20 µL·s−1 fluid flow for 20 seconds, as indicated by the shaded areas. (D) Summary data for experiments of the type shown in panels A-C. Presented is the peak (maximum [max]) change in Vm of WT, PIEZO1WT/M-R, and PIEZO1M-R/M-R RBC counts after exposure to flow and Vm at 1 to 5 minutes after flow. Averaged data are displayed as means ± standard deviation, and each data point is shown. WT: n = 20 (max, 1 minute), 16 (2 minutes), 13 (3 minutes), and 9 (4 and 5 minutes); PIEZO1WT/M-R: n = 13 (max), 12 (1 minute), 11 (2 minutes), 10 (3 minutes), and 9 (4 and 5 minutes); and PIEZO1M-R/M-R: n = 14 (max, 1 minute), 13 (2 minutes), 11 (3 minutes), 9 (4 minutes), and 8 (5 minutes). (E) As in panel D, except with 2.5 μM GsMTx4 in the extracellular solution. WT: n = 5 (max, 1 minute, 2 minutes, 3 minutes), 4 (4 minutes), and 3 (5 minutes); PIEZO1WT/M-R: n = 7 (max, 1 minute), 6 (2 minutes), 4 (3 and 4 minutes), 3 (5 minutes); and PIEZO1M-R/M-R: n = 8 (max), 6 (1 minute), 5 (2 minutes), 4 (3 minutes), and 3 (4 and 5 minutes). (F-H) Ionic current recordings obtained using the perforated-patch technique in whole-cell, voltage-clamp mode applied to freshly isolated RBCs from WT (F), PIEZO1WT/M-R (G), and PIEZO1M-R/M-R mice (H). RBCs were exposed to 20 µL·s−1 fluid flow for 20 seconds, as indicated by the shaded areas. Holding voltage was −80 mV. (I) Summary data for experiments of the type shown in panels F-H. Presented is the peak (max) change in current (ΔI) of WT, PIEZO1WT/M-R RBCs after exposure to flow and then the ΔI at 1 and 2 minutes after flow. Averaged data are means ± standard deviation, and individual data points are shown. WT: n = 23 (max, 1 minute) and 15 (2 minutes); PIEZO1WT/M-R: n = 12 (max, 1 minute) and 10 (2 minutes); and PIEZO1M-R/M-R: n = 18 (max), 15 (1 minute), and 11 (2 minutes). (J) As in panel I, except with 2.5 μM GsMTx4 in the extracellular solution. WT: n = 5 (max, 1 minute, 2 minutes); PIEZO1WT/M-R: n = 4 (max, 1 minute), 3 (2 minutes); and PIEZO1M-R/M-R: n = 4 (max, 1 minute, 2 minutes). Statistical analysis by one-way ANOVA with Bonferroni’s post hoc test indicated: (D,I) significant decay of the WT (**P < .01; *** P < .001) but not mutant RBC responses; (D) significant increase in the peak response in PIEZO1M-R/M-R RBCs compared with WT (P < .05); (E) no significant effects of flow in WT or PIEZO1WT/M-R, but significant hyperpolarization at 1 minute compared with the peak in PIEZO1M-R/M-R (P < .01); and (J) no significant effects of flow in WT or PIEZO1WT/M-R but significant outward current at 1 and 2 minutes compared with peak in PIEZO1M-R/M-R (P < .001).
Slow kinetics of RBC PIEZO1 mice and slow recovery in PIEZO1WT/M-R and PIEZO1M-R/M-R mice. (A-C) Membrane potential (Vm) recordings obtained using the perforated-patch, whole-cell mode applied to freshly isolated RBCs from WT (A), PIEZO1WT/M-R (B), and PIEZO1M-R/M-R (C) mice. RBCs were exposed to 20 µL·s−1 fluid flow for 20 seconds, as indicated by the shaded areas. (D) Summary data for experiments of the type shown in panels A-C. Presented is the peak (maximum [max]) change in Vm of WT, PIEZO1WT/M-R, and PIEZO1M-R/M-R RBC counts after exposure to flow and Vm at 1 to 5 minutes after flow. Averaged data are displayed as means ± standard deviation, and each data point is shown. WT: n = 20 (max, 1 minute), 16 (2 minutes), 13 (3 minutes), and 9 (4 and 5 minutes); PIEZO1WT/M-R: n = 13 (max), 12 (1 minute), 11 (2 minutes), 10 (3 minutes), and 9 (4 and 5 minutes); and PIEZO1M-R/M-R: n = 14 (max, 1 minute), 13 (2 minutes), 11 (3 minutes), 9 (4 minutes), and 8 (5 minutes). (E) As in panel D, except with 2.5 μM GsMTx4 in the extracellular solution. WT: n = 5 (max, 1 minute, 2 minutes, 3 minutes), 4 (4 minutes), and 3 (5 minutes); PIEZO1WT/M-R: n = 7 (max, 1 minute), 6 (2 minutes), 4 (3 and 4 minutes), 3 (5 minutes); and PIEZO1M-R/M-R: n = 8 (max), 6 (1 minute), 5 (2 minutes), 4 (3 minutes), and 3 (4 and 5 minutes). (F-H) Ionic current recordings obtained using the perforated-patch technique in whole-cell, voltage-clamp mode applied to freshly isolated RBCs from WT (F), PIEZO1WT/M-R (G), and PIEZO1M-R/M-R mice (H). RBCs were exposed to 20 µL·s−1 fluid flow for 20 seconds, as indicated by the shaded areas. Holding voltage was −80 mV. (I) Summary data for experiments of the type shown in panels F-H. Presented is the peak (max) change in current (ΔI) of WT, PIEZO1WT/M-R RBCs after exposure to flow and then the ΔI at 1 and 2 minutes after flow. Averaged data are means ± standard deviation, and individual data points are shown. WT: n = 23 (max, 1 minute) and 15 (2 minutes); PIEZO1WT/M-R: n = 12 (max, 1 minute) and 10 (2 minutes); and PIEZO1M-R/M-R: n = 18 (max), 15 (1 minute), and 11 (2 minutes). (J) As in panel I, except with 2.5 μM GsMTx4 in the extracellular solution. WT: n = 5 (max, 1 minute, 2 minutes); PIEZO1WT/M-R: n = 4 (max, 1 minute), 3 (2 minutes); and PIEZO1M-R/M-R: n = 4 (max, 1 minute, 2 minutes). Statistical analysis by one-way ANOVA with Bonferroni’s post hoc test indicated: (D,I) significant decay of the WT (**P < .01; *** P < .001) but not mutant RBC responses; (D) significant increase in the peak response in PIEZO1M-R/M-R RBCs compared with WT (P < .05); (E) no significant effects of flow in WT or PIEZO1WT/M-R, but significant hyperpolarization at 1 minute compared with the peak in PIEZO1M-R/M-R (P < .01); and (J) no significant effects of flow in WT or PIEZO1WT/M-R but significant outward current at 1 and 2 minutes compared with peak in PIEZO1M-R/M-R (P < .001).
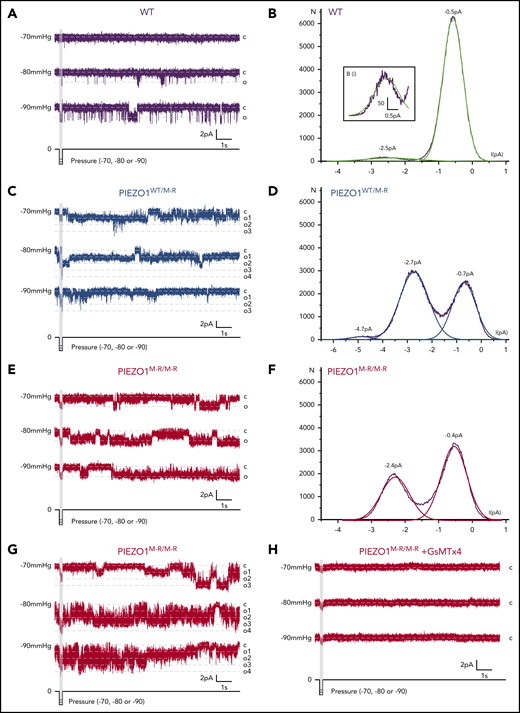
To investigate more closely, we sought single channel data by recording from cell-attached membrane patches under voltage-clamp and applying short pressure pulses to the patch pipette to increase membrane tension. Occasionally, there was only 1 unitary current level, suggesting only 1 channel in the patch (Figure 2A,E), but in most cases, there were multiple channels (eg, Figure 2C,G). PIEZO1 channels were identified by the signature unitary current amplitude1 (Figure 2B,D,F; supplemental Figure 11). In the WT exemplar shown, activity was evoked by the −80 and −90 mm Hg pressure pulses, but afterward, there were only infrequent openings, suggesting that this channel mostly deactivated (Figure 2A). The rarity of channel opening was seen particularly in the amplitude histogram, which indicated low frequency at −2.5 pA (channel open) relative to that at −0.5 pA (channel closed; Figure 2B). In contrast, in PIEZO1WT/M-R and PIEZO1M-R/M-R RBCs, there was identical unitary current amplitude but remarkably higher activity after termination of pressure pulses (Figure 2C-F). Histogram analysis highlighted the difference between WT and PIEZO1WT/M-R/PIEZO1M-R/M-R, because the frequency of open-state activity (eg, at −2.5, −2.7, and −2.4 pA) was high relative to the closed-state frequency (ie, at −0.5, −0.7, and −0.4 pA; Figure 2D,F, compare with Figure 2B). Patches containing multiple PIEZO1M-R/M-R channels in the same patch also showed persistent high activity (Figure 2G). Summary analysis of multichannel activity also supported the conclusion that persistent activity was greater in PIEZO1WT/M-R/PIEZO1M-R/M-R (supplemental Figure 12). Unitary current events were abolished by GsMTx4 (Figure 2H).
Failure of deactivation in PIEZO1WT/M-R and PIEZO1M-R/M-R single channels. Data are for single-channel activity measured by the cell-attached patch technique applied to freshly isolated RBCs of WT (A-B), PIEZO1WT/M-R (C-D), and PIEZO1M-R/M-R (E-H) mice. A fast pressure-clamp system applied brief (200 ms) negative pressure pulses to the patches, as indicated below the original exemplar current traces (A,C,E,G,H). Dashed horizontal lines indicate current levels for closed channels (c) and open channels (o, or o1-4 for multiple channel openings in panels C and G). Constant voltage of +80 mV was applied to the patch pipette. (A,E) Data are considered to be for patches each containing only 1 PIEZO1 channel; (C,G) patches contain multiple PIEZO1 channels. (B,D,F) Amplitude histogram analysis for the exemplar traces, showing plots of the frequency of detection of events (N) at the current amplitudes indicated on the x-axis, where zero current indicates no current flowing in the circuit. The closed channel state appears as a small negative current peaking at −0.5 (B), −0.7 (D), and −0.4 pA (F). (Bi) The small amount of open channel activity at −2.5 pA is shown in an expansion. Raw data are shown with superimposed Gaussian fits in green (WT), blue (PIEZO1WT/M-R), and red (PIEZO1M-R/M-R). Representative of n = 6, 5, and 4 for WT, PIEZO1WT/M-R, and PIEZO1M-R/M-R, respectively. (H) An exemplar recording from a PIEZO1M-R/M-R RBC patch with 2.5 μM GsMTx4 in the extracellular solution (n = 5).
Failure of deactivation in PIEZO1WT/M-R and PIEZO1M-R/M-R single channels. Data are for single-channel activity measured by the cell-attached patch technique applied to freshly isolated RBCs of WT (A-B), PIEZO1WT/M-R (C-D), and PIEZO1M-R/M-R (E-H) mice. A fast pressure-clamp system applied brief (200 ms) negative pressure pulses to the patches, as indicated below the original exemplar current traces (A,C,E,G,H). Dashed horizontal lines indicate current levels for closed channels (c) and open channels (o, or o1-4 for multiple channel openings in panels C and G). Constant voltage of +80 mV was applied to the patch pipette. (A,E) Data are considered to be for patches each containing only 1 PIEZO1 channel; (C,G) patches contain multiple PIEZO1 channels. (B,D,F) Amplitude histogram analysis for the exemplar traces, showing plots of the frequency of detection of events (N) at the current amplitudes indicated on the x-axis, where zero current indicates no current flowing in the circuit. The closed channel state appears as a small negative current peaking at −0.5 (B), −0.7 (D), and −0.4 pA (F). (Bi) The small amount of open channel activity at −2.5 pA is shown in an expansion. Raw data are shown with superimposed Gaussian fits in green (WT), blue (PIEZO1WT/M-R), and red (PIEZO1M-R/M-R). Representative of n = 6, 5, and 4 for WT, PIEZO1WT/M-R, and PIEZO1M-R/M-R, respectively. (H) An exemplar recording from a PIEZO1M-R/M-R RBC patch with 2.5 μM GsMTx4 in the extracellular solution (n = 5).
The data suggest that RBCs provide a special environment for PIEZO1 that disables or greatly slows the rapid inactivation mechanism and confers the importance of deactivation, which is a different mechanism from that of inactivation. In future studies, it will be interesting to determine how the rapid inactivation gate is disabled or slowed in RBCs. Our work on endothelial PIEZO1 has suggested that sphingomyelinase can cause such an effect.23 Sphingomyelinase is known to regulate RBC membrane structure24 and adhesion of eryptotic RBCs to endothelial cells.25 It will also be interesting to determine the molecular mechanism of deactivation in PIEZO1, its sensitivity to other DHS mutations in the RBC context, and the potential for correction by small molecules. Such studies could have multiple benefits for better understanding RBC homeostasis and DHS, and could enable better appreciation of the mechanical biology of other blood-borne cells that normally live in an environment of dynamic shear stress.
Source data is provided online in the supplementary Data.
The online version of this article contains a data supplement.
Acknowledgments
The authors thank the Blood Sciences Laboratory at Leeds General Infirmary for analysis of blood samples.
This work was supported by Wellcome Trust Investigator grant 110044/Z/15/Z and British Heart Foundation programme grant RG/17/11/33042 (D.J.B.), an Academy of Medical Sciences and Wellcome Trust Springboard Award grant SBF002\1031 (A.C.K.), and a British Heart Foundation Studentship grant FS/16/44/32356 (E.L.E.).
Authorship
Contribution: E.L.E., O.V.P., M.J.L, F.M., and L.L. performed experiments and data analyses; O.V.P. performed and analyzed the patch-clamp recordings; D.D.V. and A.C.K. generated the molecular models; M.J.L., N.E.H., and A.A. generated the PIEZO1M-R/M-R mouse; T.S.F. led the mouse breeding strategy; G.P. provided technical support; E.L.E., O.V.P., M.J.L., and D.J.B. designed the research; and D.J.B. raised funds to support the work and wrote the paper with the support of all authors.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: David J. Beech, Leeds Institute of Cardiovascular and Metabolic Medicine, School of Medicine, LIGHT Building, Clarendon Way, University of Leeds, Leeds LS2 9JT, United Kingdom; e-mail: d.j.beech@leeds.ac.uk; Melanie J. Ludlow, Leeds Institute of Cardiovascular and Metabolic Medicine, School of Medicine, LIGHT Building, Clarendon Way, University of Leeds, Leeds LS2 9JT, United Kingdom; e-mail m.j.ludlow@leeds.ac.uk; and Antreas C. Kalli, Leeds Institute of Cardiovascular and Metabolic Medicine, School of Medicine, LIGHT Building, Clarendon Way, University of Leeds, Leeds LS2 9JT, United Kingdom; e-mail: a.kalli@leeds.ac.uk.
REFERENCES
Author notes
E.L.E. and O.V.P. contributed equally to this study.

![Slow kinetics of RBC PIEZO1 mice and slow recovery in PIEZO1WT/M-R and PIEZO1M-R/M-R mice. (A-C) Membrane potential (Vm) recordings obtained using the perforated-patch, whole-cell mode applied to freshly isolated RBCs from WT (A), PIEZO1WT/M-R (B), and PIEZO1M-R/M-R (C) mice. RBCs were exposed to 20 µL·s−1 fluid flow for 20 seconds, as indicated by the shaded areas. (D) Summary data for experiments of the type shown in panels A-C. Presented is the peak (maximum [max]) change in Vm of WT, PIEZO1WT/M-R, and PIEZO1M-R/M-R RBC counts after exposure to flow and Vm at 1 to 5 minutes after flow. Averaged data are displayed as means ± standard deviation, and each data point is shown. WT: n = 20 (max, 1 minute), 16 (2 minutes), 13 (3 minutes), and 9 (4 and 5 minutes); PIEZO1WT/M-R: n = 13 (max), 12 (1 minute), 11 (2 minutes), 10 (3 minutes), and 9 (4 and 5 minutes); and PIEZO1M-R/M-R: n = 14 (max, 1 minute), 13 (2 minutes), 11 (3 minutes), 9 (4 minutes), and 8 (5 minutes). (E) As in panel D, except with 2.5 μM GsMTx4 in the extracellular solution. WT: n = 5 (max, 1 minute, 2 minutes, 3 minutes), 4 (4 minutes), and 3 (5 minutes); PIEZO1WT/M-R: n = 7 (max, 1 minute), 6 (2 minutes), 4 (3 and 4 minutes), 3 (5 minutes); and PIEZO1M-R/M-R: n = 8 (max), 6 (1 minute), 5 (2 minutes), 4 (3 minutes), and 3 (4 and 5 minutes). (F-H) Ionic current recordings obtained using the perforated-patch technique in whole-cell, voltage-clamp mode applied to freshly isolated RBCs from WT (F), PIEZO1WT/M-R (G), and PIEZO1M-R/M-R mice (H). RBCs were exposed to 20 µL·s−1 fluid flow for 20 seconds, as indicated by the shaded areas. Holding voltage was −80 mV. (I) Summary data for experiments of the type shown in panels F-H. Presented is the peak (max) change in current (ΔI) of WT, PIEZO1WT/M-R RBCs after exposure to flow and then the ΔI at 1 and 2 minutes after flow. Averaged data are means ± standard deviation, and individual data points are shown. WT: n = 23 (max, 1 minute) and 15 (2 minutes); PIEZO1WT/M-R: n = 12 (max, 1 minute) and 10 (2 minutes); and PIEZO1M-R/M-R: n = 18 (max), 15 (1 minute), and 11 (2 minutes). (J) As in panel I, except with 2.5 μM GsMTx4 in the extracellular solution. WT: n = 5 (max, 1 minute, 2 minutes); PIEZO1WT/M-R: n = 4 (max, 1 minute), 3 (2 minutes); and PIEZO1M-R/M-R: n = 4 (max, 1 minute, 2 minutes). Statistical analysis by one-way ANOVA with Bonferroni’s post hoc test indicated: (D,I) significant decay of the WT (**P < .01; *** P < .001) but not mutant RBC responses; (D) significant increase in the peak response in PIEZO1M-R/M-R RBCs compared with WT (P < .05); (E) no significant effects of flow in WT or PIEZO1WT/M-R, but significant hyperpolarization at 1 minute compared with the peak in PIEZO1M-R/M-R (P < .01); and (J) no significant effects of flow in WT or PIEZO1WT/M-R but significant outward current at 1 and 2 minutes compared with peak in PIEZO1M-R/M-R (P < .001).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/136/1/10.1182_blood.2019004174/4/m_bloodbld2019004174f1.png?Expires=1769090583&Signature=4V-XHWIyUZD17w9PRU8MuWqQlB3xdXexCAV67pm6unumwQNU5KMBD~jqMS2G2lNqwgipLQKCslvprucoga9pytKQ3HNGn2754G2rEB-UIvX8bjFzKvrovggQw-5cRdFdlSYm2EVg4MBh8lgzTqRdYCjtu5isNzC~hhrpPHcDi1PNDPspBbNVEFQhuY-Bkz~M2fulJXejUkJF6jcr7XRpvaciUOnpJF3WxlgesWe~wgEwbiR241PIfT3rDshDOveCH-qMWGQ8AI8NNmxed~M-KfRFHL3wTd6RkHNoegX4ZUgsIibDPv~G9oHtsjX2iuJt0Z~gDjM7RZ5f6LXtPKq4Kw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)