In this issue of Blood, Arezes et al report on the development of a monoclonal antibody that neutralizes the activity of the iron-regulatory erythrokine erythroferrone (ERFE). In a mouse model of β-thalassemia intermedia, the monoclonal antibody increased hemoglobin levels and decreased serum iron concentration and systemic iron overload.1
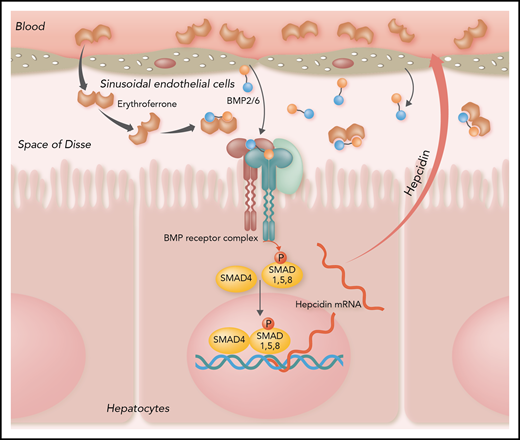
Shown is a current model of the mechanism of action of ERFE. ERFE binds and sequesters heterodimeric BMP2/6 secreted by sinusoidal endothelial cells into the space of Disse and thereby prevents BMP2/6 from binding to the hepatocyte BMP receptor. Diminished BMP receptor signaling then decreases hepcidin gene transcription and protein secretion.
Shown is a current model of the mechanism of action of ERFE. ERFE binds and sequesters heterodimeric BMP2/6 secreted by sinusoidal endothelial cells into the space of Disse and thereby prevents BMP2/6 from binding to the hepatocyte BMP receptor. Diminished BMP receptor signaling then decreases hepcidin gene transcription and protein secretion.
During maximally stimulated erythropoiesis (eg, after hemorrhage), hemoglobin synthesis increases up to 10-fold and must be supported by a comparable increase in iron delivery from stores and diet. This response starts within hours after the precipitating event and develops without an appreciable change in serum iron concentration. Five years ago, ERFE2 was discovered as a new hormone secreted by erythropoietin-stimulated erythroblasts, and it acts on the liver to suppress the production of the iron-regulatory hormone hepcidin. Circulating ERFE levels increase after hemorrhage or in response to stimulation with erythropoietin, and they help maintain stable plasma iron concentrations by mobilizing iron to meet the requirements of erythropoiesis.2
Remarkably, plasma ERFE concentrations are greatly increased in anemias with ineffective erythropoiesis,3,4 probably because of ERFE secretion by a greatly expanded population of mostly dead-end erythroblasts in both the marrow and at extramedullary sites. Plasma ERFE concentrations seem to be inversely related to those of hepcidin, consistent with the proposed suppressive effect of ERFE on hepcidin. In a mouse model of β-thalassemia intermedia, genetic ablation of ERFE reversed the suppression of hepcidin and partly prevented iron accumulation.4
The mechanism of action of ERFE has been a mystery, and multiple searches for ERFE receptors turned up no information. ERFE belongs to the C1q-TNF-α–related protein family, characterized by an N-terminal extended region containing a variably long collagen-like multimerization motif and a C-terminal globular head that structurally resembles that of TNF-α or the C1q complement protein. The article by Arezes et al provides 2 important insights. First, they documented that the bioactive segment of ERFE is in the N-terminal extended region, an observation also made independently by another group.5 Importantly, they also found that ERFE seems to act directly on bone morphogenetic protein (BMP) signaling,6 the main pathway by which the transcription of the hepcidin gene is regulated. Unexpectedly, the N terminus of ERFE binds the BMP receptor ligands BMP2 and BMP6, which had been implicated as being essential for hepcidin regulation. Here, the relevant physiological ligand may be the BMP2/6 heterodimer, which could be the species entrapped by ERFE (see figure), as suggested by others.7 The avidity of the interaction between ERFE and these BMPs may be further strengthened by the multimeric structure of ERFE (in this regard, behaving similarly to adiponectin8 ), with multimerization promoted by the interactions between the globular C-terminal regions. Although the existence of a receptor for ERFE is not excluded by these observations, the proposed mechanism of action explains all relevant findings to date.
Unlike its downstream target hepcidin, which circulates at nanomolar concentrations, ERFE is active at picomolar concentrations, making it an ideal target for monoclonal antibody therapy. Arezes et al developed such antibodies, appropriately targeting the bioactive N terminus of ERFE, and demonstrated that these antibodies neutralized the hepcidin-suppressive activity of ERFE in a hepatocyte cell line as well as in mice. Importantly, these antibodies exerted a beneficial effect in the murine β-thalassemia intermedia model, in which they ameliorated anemia, reversed the suppression of hepcidin, decreased serum iron concentrations, and diminished hepatic iron overload. Supporting the feasibility of this approach, largely similar results were also obtained by another group with a different set of antibodies.9,10 A puzzling effect of anti-ERFE antibodies is that they improve anemia in thalassemic mice, an effect not seen when the ERFE-encoding Erfe gene was ablated in these mice.4 It is possible that the complete loss of ERFE during development elicits some form of compensation not seen when ERFE is neutralized by antibodies in adult mice.
In view of the broad importance of BMP signaling in many organs and tissues, systemic inhibition of BMP signaling by ERFE in iron-loading anemias may contribute to the many nonhematologic manifestations of these diseases, raising hope that some of these may also respond to anti-ERFE therapy. Anti-ERFE antibodies are expected to be well tolerated in humans because ERFE, at least in mice, is a stress hormone that is not essential for health or survival. The addition of yet another preclinical candidate to the rapidly widening pipeline of treatment options for thalassemias and other anemias with ineffective erythropoiesis is a cause for optimism.
Conflict-of-interest disclosure: T.G. is a scientific founder and shareholder of Silarus Therapeutics and Intrinsic LifeSciences and serves as a consultant to Global Blood Therapeutics, Sierra Oncology, Ionis, American Regent, Akebia, and Regeneron. T.G. has received grant funding from Akebia/Keryx and Sierra Oncology.