In this issue of Blood, Ivanov et al1 demonstrate that the apple domain–containing proenzyme prekallikrein (PK) has single-chain proteolytic activity capable of initiating reciprocal activation with factor XII (FXII).
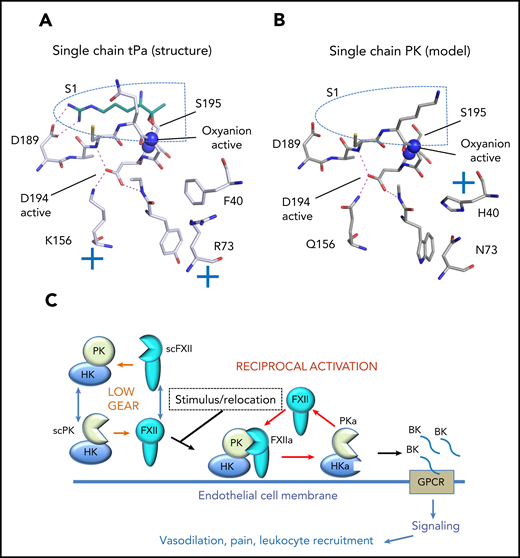
A model of the single-chain (sc) prekallikrein (scPK) structure and function is shown. (A) The single-chain tPA crystal structure (pdb:1BDA) is shown with stick diagrams of key residues around the S1 pocket (blue dotted line). The position of the oxyanion hole is indicated by blue spheres that represent the nitrogen atoms of Ser195 and Gly193. The single-chain tPa active S1 pocket reveals Asp194 is stabilized by a salt bridge formed with Lys156. The cyan stick figure represents the side chain of the arginine P1 residue of the tPa inhibitor dansyl-Glu-Gly-Arg-chloromethyl-ketone. (B) Homology model of the S1 pocket of scPK based on the tPA crystal structure where Gln156 forms a hydrogen bond to the Asp194 carboxylate group. (C) The low gear of weak proteolytic activity of the contact system consists of both scPK and scFXII, which constitutively consume each other. Activation of the system requires a stimulus and location to the correct cell membrane to form PKa, FXIIa, and bradykinin (BK).
A model of the single-chain (sc) prekallikrein (scPK) structure and function is shown. (A) The single-chain tPA crystal structure (pdb:1BDA) is shown with stick diagrams of key residues around the S1 pocket (blue dotted line). The position of the oxyanion hole is indicated by blue spheres that represent the nitrogen atoms of Ser195 and Gly193. The single-chain tPa active S1 pocket reveals Asp194 is stabilized by a salt bridge formed with Lys156. The cyan stick figure represents the side chain of the arginine P1 residue of the tPa inhibitor dansyl-Glu-Gly-Arg-chloromethyl-ketone. (B) Homology model of the S1 pocket of scPK based on the tPA crystal structure where Gln156 forms a hydrogen bond to the Asp194 carboxylate group. (C) The low gear of weak proteolytic activity of the contact system consists of both scPK and scFXII, which constitutively consume each other. Activation of the system requires a stimulus and location to the correct cell membrane to form PKa, FXIIa, and bradykinin (BK).
Most cars have a low-gear mode that enables rapid acceleration from a standing start. Ivanov et al describe a low-gear mode for plasma protease PK in the form of weak single-chain enzyme activity. This finding builds on work from the same group that demonstrated single-chain activity for coagulation FXII. Thus, both enzymes have background proteolytic activity inherent to the intact polypeptide that was traditionally described as an inactive zymogen. This explains why the 2 enzymes appear to consume each other constitutively, and a depletion of PK gives rise to an increased plasma concentration of FXII.2 High-gear activation of the contact system likely involves additional factors, such as polyphosphate coupled with relocation to the appropriate cell membrane. Once PK, FXII are converted by cleavage of the activation loop to generate 2 chain PKa and FXIIa, the high gear required for bradykinin production and initiation of the intrinsic pathway is engaged.
PK is an apple domain–containing protease closely related to factor XI (FXI) and thus has a different structure than FXII, which most closely resembles hepatocyte growth factor (HGFA). FXII also clusters with plasminogen activators tPa and uPa in terms of overall sequence identity. The first example of single-chain proteolytic activity was characterized for tPa. The remarkable crystal structure for tPa revealed the basis of the single-chain activity. A positive charge from the amine group of tPa residue Lys156 salt bridges the carboxylate of Asp194 to stabilize the active/open conformation of the oxyanion hole and S1 pocket (see figure panel A).3 The new data for PK provide a compelling argument that single-chain activity can be generalized more widely to serine proteinases in the trypsin and chymotrypsin family. How does the low-gear mechanism work for FXII and PK? These enzymes do not have Lys156, but Gln appears in an equivalent position for FXII, PK as well as chymotrypsin, thrombin, and FXI. A model of the PK structure based on tPA reveals that Gln156 can coordinate the Asp194 carboxylate and stabilizes the open conformation of the enzyme through hydrogen bonding (see figure panel B).
Proteases factor VII (FVII) and FVII activating protease have Met156 and Leu156, respectively, and these hydrophobic residues would not be expected to provide a similar stabilization of Asp194 as Lys156/Gln156, which is consistent with the observed higher zymogenicity for these enzymes. Mutagenesis studies that replaced FVII Met156 with Gln resulted in an increase in proteolytic activity.4 Proteases thus have a variety of ways to modulate their single-chain activity to a minimum. Factor X has Lys156, and HGFA has Arg156; these proteases have not been characterized as having high levels of single-chain activity like tPa. In this respect, it is interesting that Ivanov et al also demonstrated that FXI does not have single-chain activity toward its substrate factor IX (FIX) despite having Gln156; so how is this achieved? One mechanism is to directly influence the local environment of Asp194 and “pull” it toward the zymogen state by addition of a positive charge to counter the influence of Lys156/Gln156. It was previously demonstrated for tPa that substitution of residue Phe40 for His can reduce single-chain activity. The His40 side chain directly stabilized Asp194, forming an Asp194-His40-Ser32 network termed the zymogen triad, which was originally observed in the chymotrypsinogen structure. The FXII sequence has Phe40, and thus, no zymogen triad exists; however, a crystal structure revealed direct contact between residues Asp194-Arg73 occurs and could be an alternative to stabilize a zymogen-like conformation.5 FXI has an Asp194-His40-Thr32 zymogen triad but so does PK, so this is not the complete explanation for the lack of single-chain activity in FXI. A second possibility is that the burial of FXI substrate-binding exosites on the apple domains can modulate the single-chain activity.6 Thus, in the zymogen, FXI cannot recruit substrate FIX without cleavage of the activation loop to release the exosite in the apple 3 domain. In this case, an extra layer of conformational regulation smothers the FXI single-chain activity to prevent inappropriate cleavage.
Single-chain activity may have an evolutionary origin in the precursors of modern regulatory proteases, which were simpler in design, lacking an activation loop and N-terminal ancillary domains. The functional significance of the single-chain activity is evident for PK and FXII because this can drive autoactivation and stimulate a cascade without the requirement of an exogenous protease to cleave the activation loop. Thus, PK can be activated by a nonproteolytic mechanism via prolyl-carboxypeptidase7 or heat shock protein 908 without the need for FXII. Perturbation of the PK protein structure is sufficient to release the clutch and engage the single-chain low-gear PK activity, which goes on to initiate autoactivation and generate PKa/FXIIa/bradykinin (see figure panel C). The unusual nature of PK is that, unlike most proteases, it circulates bound to its substrate kininogen (HK). Structural studies have suggested that PK is bound to HK at a location remote to the active site, which may prevent single-chain proteolytic cleavage.6
The single-chain activity of PK and FXII changes our perception of how the network of connections between the contact system and the intrinsic pathway is initiated and how to inhibit them. The recent report of PKa activating FIX in the presence of polyphosphate illustrates in certain contexts that these 2 systems are more closely synchronized than previously thought.9 The amount and location of thrombin and fibrin required for an innate immune response likely differ compared with hemostasis. Although polyphosphate acts as an accelerant for the reactions of the contact system and intrinsic pathway, what kick starts the contact system by drawing on the single-chain activities of FXII and PK remains unknown.
Conflict-of-interest disclosure: The author declares no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal