In this issue of Blood, Sakai et al report that the HLA alleles DRB1*08:03, DRB3/4/5*blank, DQA1*01:03, and DQB1*06:01 predisposed patients in a Japanese cohort to immune-mediated thrombotic thrombocytopenic purpura (iTTP). The alleles DRB1*15:01 and DRB5*01:01 had a protective effect. Interestingly, although these genetic risk factors for iTTP differ between Japanese and white patients, the HLA-DR molecules encoded by the main predisposing allele of both ethnic groups may bind a shared ADAMTS13 peptide. These results extend the role of the HLA system as a major genetic risk factor for iTTP.1
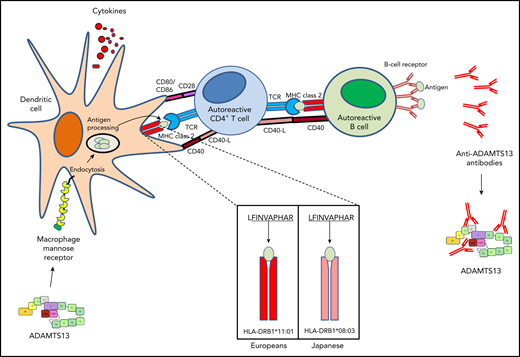
Immune response against ADAMTS13. The amino acid residues underlined are those recognized by the major histocompatibility complex (MHC) class 2 complex. CD40-L, CD40 ligand; TCR, T-cell receptor. Adapted with permission from Hrdinová et al.3
Immune response against ADAMTS13. The amino acid residues underlined are those recognized by the major histocompatibility complex (MHC) class 2 complex. CD40-L, CD40 ligand; TCR, T-cell receptor. Adapted with permission from Hrdinová et al.3
TTP is a devastating disease resulting from a severe deficiency in the von Willebrand factor (VWF)-cleaving protease ADAMTS13. This deficiency causes the accumulation of ultralarge VWF multimers in the circulation and the formation of thrombi in the microvasculature, leading to multiorgan failure and death if left untreated. In the autoimmune form of the disease (iTTP), patients develop antibodies against ADAMTS13 that enhance its clearance or inhibit its VWF processing activity. These findings provided a rationale for the use of targeted therapies, including the supply of ADAMTS13, immunomodulation, and inhibition of VWF-platelet interaction.2
Although significant progress has been made in delineating the pathophysiology of iTTP, our understanding of the immunopathogenesis of the disease is limited. The HLA system has an important role as a genetic risk factor in autoimmune diseases, and a similar association could thus be possible for iTTP.3 The crucial role of class-switched high-affinity anti-ADAMTS13 antibodies in iTTP pathophysiology implies cooperation between T and B lymphocytes. Moreover, activation of ADAMTS13-specific CD4+ T cells requires recognition of ADAMTS13 peptides bound to HLA molecules by T-cell receptors, thereby implicating HLA molecules as predisposing factors for iTTP.3 This hypothesis has proven fruitful, \and studies to determine the mechanisms involved in the loss of tolerance of ADAMTS13 in white adults, as well as pediatric patients have identified HLA class 2 locus DRB1*11 and DQB1*03 alleles as genetic risk factors and DRB1*04 as a protective allele. The evaluation of HLA haplotypes has also revealed a higher frequency of DQB1*03-DRB1*11 haplotype in iTTP patients than in population controls.4-7 Further evidence for the involvement of the HLA-DRB1*11 and HLA-DQB1*03 haplotype in the development of iTTP was provided from a case of familial iTTP in 2 first-degree relatives (mother and daughter) who both carried HLA-DRB1*11:01/DRB1*11:04 and the linked HLA-DQB1*03 allele (reviewed by Hrdinová et al3 ). Other protective (DRB1*07-DQB1*02 and DRB1*13-DQB1*06) or predisposing (DRB1*15DQB1*06) haplotypes for iTTP8 have also been identified. Lastly, a single-nucleotide polymorphism (rs6903608) in the HLA locus that affects expression of a number of MHC class 2 subunits has been linked to the onset of iTTP, further implicating HLA in the pathogenesis of iTTP.9 Activation of ADAMTS13-specific CD4+ T cells requires uptake of ADAMTS13-derived peptides on HLA molecules by antigen-presenting cells. These peptides were found to have core sequences originating from the CUB2 domain of ADAMTS13.10 Interestingly, these findings on the role of HLA in iTTP immunopathogenesis could explain the ethnic disparities observed in this disease; indeed, the protective allele DRB1*04 has a naturally low prevalence in people from Africa and the West Indies, which could at least in part account for the overrepresentation of these ethnic groups in most iTTP registries.3 Until now, whether the HLA risk factors identified in patients of European descent were also relevant in Asians was unknown.
Sakai et al studied 10 HLA loci using next-generation sequencing in 52 Japanese patients with iTTP.1 By comparing their results with those of a Japanese control group, they identified predisposing and protective HLA alleles significantly associated with iTTP, with odds ratios ranging from 3.06 to 2.25. The HLA alleles they identified were different from those observed in patients of European descent. Interestingly, despite this apparent discrepancy between the 2 ethnic groups, they provided evidence that HLA-DR molecules encoded balert_message_vary the main predisposing allele DRB1*08:03 could bind an ADAMTS13 peptide in the CUB2 domain to a sequence that is very close to the peptide bound by DRB1*11, the counterpart predisposing allele in whites. Indeed, these 2 peptides were found to differ only by the shift of 1 amino acid residue (see figure). Taken together, these findings support the consistent view that the HLA system represents a major, universal risk factor involved in the loss of tolerance of the immune system toward ADAMTS13.
This study has some limitations. First, the analysis is limited by the small number of patients. Second, the binding studies between HLA-DR molecules and the CUB2-derived peptides are based only on the results of in silico analysis, calling for further in vitro MHC peptide experiments to definitely demonstrate this finding.
Despite these limitations, the results reported by Sakai et al, showing the key role of the HLA system in iTTP pathophysiology, add a new piece to the complex puzzle of iTTP immunopathogenesis. For example, forthcoming studies in the field should address how the alleles of the HLA system identified as risk factors, which are frequent in the general healthy population, are capable of inducing the production of pathogenic anti-ADAMTS13 antibodies (and thereby iTTP) in only a very small number of cases. In this way, studies should unravel the interaction between modifying factors, such as sex, obesity, and hormones, and the genetic risk factors of iTTP. We are also at an opportune time to explore a more comprehensive landscape of the genetic risk factors of the disease (ie, besides the HLA system), with the use of approaches including genome-wide association studies and whole-genome sequencing. Lastly, the development of a recombinant form of ADAMTS13 opens a new therapeutic era in the field. Interactions of recombinant ADAMTS13 with the immune systems of iTTP patients will require intensive investigation, especially with regard to potential immunogenicity. Better understanding of iTTP immunopathogenesis is therefore crucial to provide a basis for the development of novel therapeutic approaches to restore immune tolerance toward ADAMTS13 and thereby better prevent disease refractoriness and relapse.
Conflict-of-interest disclosure: The author declares no competing financial interests.