In this issue of Blood, Grants et al have identified connections between reduced expression of microRNA-146a (miR-146a), increased inflammation, impaired hematopoietic stem cell (HSC) quiescence, and a poor prognosis for acute myeloid leukemia (AML).1 miR-146a was originally identified as an anti-inflammatory microRNA that targets signaling proteins, which mediate inflammatory responses.2,3 Deletion of miR-146a in mice phenocopies many aspects of age-dependent inflammation,2,4 termed “inflammaging.”
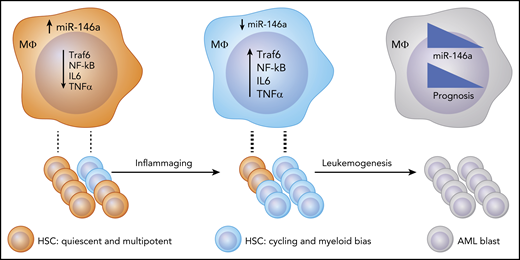
Proposed model: as a consequence of inflammaging, miR-146a levels are reduced, and inflammatory cytokine production is increased in hematopoietic cells, including macrophages. Consequently, these cytokines promote HSC proliferation and differentiation into stem cells with a myeloid and, potentially, leukemic bias. This state contributes to malignant transformation by some cell clones. Upon transformation to AML, miR-146a expression negatively correlates with prognosis.
Proposed model: as a consequence of inflammaging, miR-146a levels are reduced, and inflammatory cytokine production is increased in hematopoietic cells, including macrophages. Consequently, these cytokines promote HSC proliferation and differentiation into stem cells with a myeloid and, potentially, leukemic bias. This state contributes to malignant transformation by some cell clones. Upon transformation to AML, miR-146a expression negatively correlates with prognosis.
Inflammaging impacts a plethora of human disease conditions, including obesity, diabetes, arthritis, heart disease, and cancer.5 However, the molecular mechanisms underlying this chronic pathological state, and how they contribute to blood cancers, remain poorly understood. In this study, the authors identify low miR-146a as a poor prognostic marker in AML and find its expression to be decreased in older patients, as well as in aging mice (see figure). The authors then use MiR-146a−/− mice, which develop myeloid malignancies in some cases,2,6 to carefully assess the impact of inflammaging on HSC phenotypes utilizing a variety of functional and state-of-the-art genomics approaches to monitor single cells.
Previous work has demonstrated that HSCs from miR-146a–deficient mice are dysfunctional.2,6 The current study clarifies that this is due to alterations in epigenetic modifications that activate inflammaging gene expression programs. These epigenetic and gene expression patterns reflect the underlying heterogeneity of the HSC pool. The composition of the HSC pool changes in miR-146a–deficient mice. The authors’ careful single-cell experiments show an initial “programmed” difference in cell division in miR-146a–deficient HSCs, which depletes primitive HSCs and causes an increase in myeloid progenitors, including possible leukemia stem cells.
The authors also make a convincing case that miR-146a expression is important in AML prognosis. miR-146a expression is not correlated with cytogenetic abnormalities or mutations that confer a prognostic impact. Rather, the effect of miR-146a is due to its role in regulating chronic inflammation. During aging, inflammatory changes accumulate, and there is a loss of HSC stemness and bias toward myeloid cell development.7 miR-146a appears to maintain “stemness” in the most primitive HSC compartment, and its loss in mice phenocopies the inflammaging phenotype, including a myeloid skewing. The mechanism of stemness maintenance involves suppression of inflammatory cytokines, including interleukin-6 and tumor necrosis factor-α, which are primarily produced by mature hematopoietic cells, such as macrophages, and shown to be functionally linked to miR-146a hematopoietic phenotypes. Interestingly, wild-type HSCs acquired some features of miR-146a−/− HSCs when they were cotransplanted, indicating a significant effect by factors extrinsic to the wild-type HSC and consistent with a cytokine-mediated mechanism of action. Hence, it will be interesting to determine if miR-146a is a determinant of initial leukemic transformation in conjunction with driver mutations that have been well documented.8
This interesting work also raises additional questions. Is there a difference in hematopoietic miR-146a expression between AML patients and age-matched controls? Studies in autoimmune disease have demonstrated polymorphisms in the promoter of miR-146a, which reduce its expression.9 It will be interesting to determine if this is the key to reduced miR146a expression during aging and in the setting of AML. Another open question is whether the difference in prognosis in AML is related to the direct sensitivity of AML blasts to inflammaging signaling or whether non-AML “inflammaged” HSCs are less able to recover within the bone marrow following therapy. Furthermore, the reduction in HSC function appears to be the result of both cell-intrinsic and cell-extrinsic mechanisms. The extent to which each mechanism contributes to HSC dysfunction is an exciting area for future investigation. Answers to these questions should guide future therapeutic strategies, including replenishing miR-146a, which initial evidence suggests is possible.10
Conflict-of-interest disclosure: The authors declare no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal