In this issue of Blood, Ronner and coworkers conclude that leukocytosis in patients with polycythemia vera (PV) is not a risk factor for thrombosis, but it is associated with disease evolution. To investigate these critical questions, the authors used a sophisticated statistical approach to interrogate data from a retrospective, multi-institutional cohort of 440 patients.1
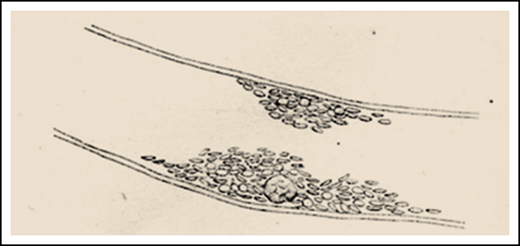
From the work of Giulio Bizzozero.11 Bizzozero, a pathologist working in Pavia, is credited for the first report of platelets being potentially implicated in thrombosis. The sketch depicts a thrombus made up of platelets on a vessel wall lining, with a leukocyte clearly visible inside. (The original figure was in an opposite orientation.)
From the work of Giulio Bizzozero.11 Bizzozero, a pathologist working in Pavia, is credited for the first report of platelets being potentially implicated in thrombosis. The sketch depicts a thrombus made up of platelets on a vessel wall lining, with a leukocyte clearly visible inside. (The original figure was in an opposite orientation.)
Theories regarding the participation of leukocytes in the pathogenesis of thrombosis are very old (see figure) and are now supported by experimental findings and clinical observations. Whether there is an affaire between leukocytes and thrombosis in patients with PV has been long debated,2 with conflicting results and opinions across numerous studies.3 The question is not trivial, especially given the clinical implications of a positive association: the latter, in fact, would elevate leukocytosis to a target for treatment to decrease the risk of thrombosis. At the same time, it would provide a reliable and easy-to-obtain biomarker of thrombosis risk and effectiveness of therapy. Leukocytosis is not formally incorporated in the conventional risk score for thrombosis in PV, that relies upon age >60 years and/or history of cardiovascular events. The supportive data for the risk score are derived from retrospective studies. However, both the European LeukemiaNet (ELN)4 recommendations and the National Comprehensive Cancer Network guidelines5 included leukocytosis >15 × 109/L and progressive leukocytosis, respectively, among the indications for initiating cytoreduction in an otherwise low-risk patient with PV. Furthermore, normalization of the leukocyte count is listed among the variables for adjudicating complete or partial remission after treatment, according to the ELN and International Working Group for Myeloproliferative Research and Treatment response criteria.6 On the other hand, leukocytosis (>10 or 15 × 109/L, depending on the particular study), together with age and venous thrombosis, predicted for transformation to post-PV myelofibrosis, which occurs with a cumulative incidence of 8% to 14% at 15 years.7 Age and leukocytosis are also major determinants of the risk of PV transforming to acute leukemia, with rate of 5% to 10% at 20 years.7 Finally, leukocytosis >15 × 109/L, together with age ≥67 years, thrombosis history, and SRSF2 mutation, was a risk factor for survival among 404 molecularly characterized PV patients.8
The basic assumption of Ronner et al is that the role of leukocytosis in PV may have been misinterpreted in retrospective series that used a single time point, rather than time-dependent data. The novelty of the work of Ronner et al relies on the use of a unique statistical approach, group-based trajectory modeling (GBTM), largely employed in social and behavioral sciences. The GBTM approach better demonstrates the evolution of an outcome over time, based on the principle that meaningful subgroups within a population exist, and follow distinctive developmental trajectories that cannot be identified a priori by the single-point measurement of individual characteristics. To apply this approach, Ronner et al used data from 440 PV patients, collected in 10 academic US institutions, who had ≥3 clinical and hematologic records available, at least 1 of which was within the last 10 years. This data set enabled the investigators to draw trajectories for the different blood cell subsets.
According to GBTM analysis, 4 clusters of patients were identified: stable leukocyte counts at 5, 10, 15, and oscillating at 35 × 109/L. The lower 2 clusters accounted for 75% of the patient population. The data set was adjusted for a number of variables, including whether the patient was receiving cytoreductive therapy in the landmark trajectory period. No association between leukocyte trajectory and thrombosis emerged, whereas leukocytosis was confirmed to be associated with disease evolution, including myelofibrosis, myelodysplasia, and acute leukemia. In addition, the study confirmed the null role of thrombocytosis on thrombosis and evolution, but generated unexpected results by showing the lack of an association with increased hematocrit and thrombosis rate. These findings contradict results of the prospective CYTO-PV trial, that randomized PV patients to different target levels of hematocrit, <45% and 45% to 50%, and showed that the former group had 4 times less thrombosis than the higher hematocrit level cohort.9 One possible explanation for these seemingly contradictory findings by Ronner et al is that they identified hematocrit clusters that were only 35%, 43%, and 47%; these low levels, which after all signify a population of well-managed PV patients from the participating centers, might have prevented the discovery of any meaningful correlation of higher hematocrit levels with thrombosis.
The study by Ronner et al is important in several respects. First, it provides an example of the potential of group-based trajectory modeling applied to large and complex databases, where the goal is to capture phenomena that are infrequent and/or delayed from the landmark start point over the course of the disease. Second, as mentioned by the authors, the multicenter database on which the study is based represents the largest US-based PV data set. This is a remarkable example of how collaboration between research institutions facilitates the creation of highly informative source of data for real-world analysis of rare clinical events in rare diseases. Third, the results of the study reinforce the dilemma of whether leukocytosis, as a surrogate marker of disease evolution potential, should prompt initiation of cytoreduction in a patient with PV.
My final take of the work of Ronner et al is that the jury still remains out on the contribution of leukocytes to the vascular events in patients with PV. It is difficult to interpret results from different retrospective cohorts that were interrogated using different statistical approaches. New drugs for treating PV are now available, with claims also of reduced rates of thrombosis,10 and other new drugs are under evaluation. These clinical trials offer a unique opportunity to prospectively test a number of disease-associated variables, including but not limited to leukocytosis, for their impact on hard clinical endpoints, beyond the cosmetic normalization of hematologic values. It is hoped, within a reasonably short time, that these studies may finally make the case for leukocytes to be, or not to be, worthy of treatment. The work of Ronner et al has rekindled a still unmet need in the modern management of patients with PV.
Conflict-of-interest disclosure: A.M.V. has received honoraria from Novartis, Celgene, and Incyte for serving on advisory boards and for lectures.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal