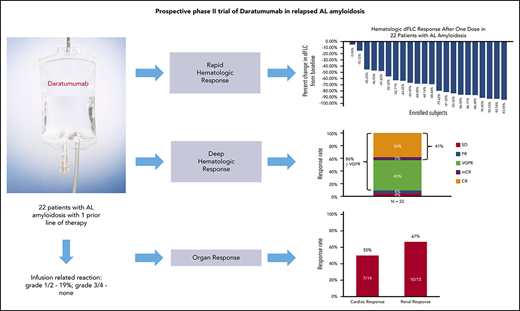
Key Points
Daratumumab is well tolerated in patients with AL amyloidosis when used with appropriate preinfusion and postinfusion medications.
Daratumumab leads to rapid and deep hematologic responses in previously treated patients with AL amyloidosis.
Abstract
Daratumumab, a monoclonal CD38 antibody, is approved in the treatment of myeloma, but its efficacy and safety in light-chain (AL) amyloidosis has not been formally studied. This prospective phase 2 trial of daratumumab monotherapy for the treatment of AL amyloidosis was designed to determine the safety, tolerability, and hematologic and clinical response. Daratumumab 16 mg/kg was administered by IV infusion once weekly for weeks 1 to 8, every 2 weeks for weeks 9 to 24, and every 4 weeks thereafter until progression or unacceptable toxicity, for up to 24 months. Twenty-two patients with previously treated AL amyloidosis were enrolled. The majority of the patients had received high-dose melphalan and stem cell transplantation and/or treatment with a proteasome inhibitor. The median time between prior therapy and trial enrollment was 9 months (range, 1-180 months). No grade 3-4 infusion-related reactions occurred. The most common grade ≥3 adverse events included respiratory infections (n = 4; 18%) and atrial fibrillation (n = 4, 18%). Hematologic complete and very-good-partial response occurred in 86% of patients. The median time to first and best hematologic response was 4 weeks and 3 months, respectively. Renal response occurred in 10 of 15 patients (67%) with renal involvement and cardiac response occurred in 7 of 14 patients (50%) with cardiac involvement. In summary, daratumumab is well tolerated in patients with relapsed AL amyloidosis and leads to rapid and deep hematologic responses and organ responses. This trial was registered at www.clinicaltrials.gov as #NCT02841033.
Introduction
Systemic light-chain (AL) amyloidosis is characterized by the overproduction of immunoglobulin light chains secreted by clonal bone marrow plasma cells that misfold and form soluble toxic aggregates. These aggregates deposit as amyloid fibrils in multiple organs causing organ dysfunction, organ failure, and death.1 Treatments for systemic AL amyloidosis are derived from established myeloma therapies, although the tolerance of these treatments in patients with AL amyloidosis is limited due to disease-related organ dysfunction. Approximately 25% to 30% of newly diagnosed patients with AL amyloidosis are eligible to receive high-dose melphalan and stem cell transplantation (HDM/SCT). This aggressive treatment leads to hematologic responses, organ responses, and prolonged survival in highly selected patients with AL amyloidosis.2 The majority of newly diagnosed patients are not eligible to receive HDM/SCT and are treated with either oral melphalan and dexamethasone3 or a combination of cyclophosphamide, bortezomib, and dexamethasone.4 Immunomodulatory agents and proteasome inhibitors of all generations have been studied and used in patients with relapsed AL amyloidosis.5-8 Despite therapeutic advances in the treatment of relapsed AL amyloidosis, it remains a challenging disease to treat, and alternative effective therapies are needed both for patients ineligible for HDM/SCT and for those with persistent or progressive disease following such treatments.
Daratumumab is a human immunoglobulin G1k monoclonal antibody targeting the CD38 surface antigen on plasma cells with proven efficacy in multiple myeloma. Daratumumab has an established role in myeloma as monotherapy as well as in combination with proteasome inhibitors and immunomodulatory agents either in the relapsed/refractory or new diagnosis settings.9 Infusion-related reactions of 48% were reported in patients treated with daratumumab as monotherapy for relapsed multiple myeloma. Although the biology of clonal plasma cells in AL amyloidosis is distinct from myeloma, clonal plasma cells in AL amyloidosis express surface CD38, providing a rationale for using daratumumab.10
There are several reports of retrospective analysis of the efficacy of daratumumab in relapsed AL amyloidosis with impressive results of rapid and deep hematologic responses.11-14 However, there are no prospective studies of the clinical trials of daratumumab in relapsed AL amyloidosis reported to date.
We designed a clinical trial to study tolerability and efficacy of daratumumab in those with relapsed AL amyloidosis. The primary objective was to determine the safety and tolerability of infusion of daratumumab, with respect to infusion-related reactions (IRRs). The secondary objectives were to assess hematologic response, clinical/organ response, and time to next treatment.
Patients and methods
Eligibility criteria
The clinical trial was approved by the Institutional Review Board of the Boston University Medical Center in accordance with federal regulations and the Declaration of Helsinki. Enrollment began in April 2017 and eligibility criteria required a tissue diagnosis of amyloidosis with evidence of an underlying clonal plasma cell dyscrasia as indicated by at least 1 of the following: a plasma cell clone in the bone marrow, detection of a monoclonal gammopathy by serum or urine immunofixation electrophoresis, or an abnormal serum free light chain concentration/ratio. Patients were required to be at least 18 years of age. All patients should have received at least 1 prior treatment regimen for AL amyloidosis that had to have been discontinued >4 weeks prior to enrollment on the trial. If prior treatment included HDM/SCT, at least 6 months must have elapsed between HDM/SCT and enrollment in the trial. Other eligibility criteria included estimated glomerular filtration rate (eGFR) by the Cockcroft-Gault equation of >20 mL/min, aspartate transaminase/alanine transaminase of <3× the upper limit of normal, total bilirubin of <2 mg/dL (bilirubin <3 mg/dL allowed if related to the Gilbert syndrome), left ventricle ejection fraction ≥ 30%, N-terminal pro-brain natriuretic peptide (NT-proBNP) of ≤8500 pg/mL, forced expiratory volume in 1 second ≥ 50% in patients with chronic obstructive pulmonary disease or chronic smokers, as well as a measure of performance status by Eastern Cooperative Oncology Group of ≤3. Patients with end-stage renal failure on dialysis were not eligible. Patients with overt multiple myeloma and bone marrow plasmacytosis of >30% were not eligible to participate.
Treatment protocol
Daratumumab (16 mg/kg IV) was administered once weekly for weeks 1 to 8, followed by every 2 weeks for weeks 9 to 24, and every 4 weeks thereafter until progression or unacceptable toxicity, for up to 24 months. The first infusion of daratumumab was given in 1000 mL of normal saline, the second infusion in 500 mL if no grade 1 or greater IRRs occurred, and all subsequent doses were administered in 500 mL. All patients received acetaminophen, diphenhydramine, loratadine, famotidine, montelukast, and methylprednisolone (100 mg for first 2 infusions and 60 mg thereafter) 30 to 60 minutes prior to infusion. Ondansetron was added after development of grade 1 nausea/vomiting in the first 2 patients. Diphenhydramine, famotidine, and methylprednisolone (40 mg) were again administered 2 hours after the start of infusion during the first 2 infusions even in the absence of an infusion reaction. Methylprednisolone 20 mg (or its equivalent) and montelukast were given 24 and 48 hours after the start of the infusion for the first 2 infusions and then was optional thereafter. All patients received prophylaxis with acyclovir for prevention of herpes zoster reactivation throughout treatment of 3 months after completion of daratumumab. Other antibacterial prophylaxis was not used during protocol-directed treatment.
Dose modifications
Dose delays and modification were allowed based on toxicity as described in the next section. In all patients experiencing such toxicities, an attempt was made to distinguish between drug toxicity, associated medical illness, or disease-related symptomology. Dose delay was the primary method for managing daratumumab-related toxicities.
Infusion reactions of any grade led to the temporary interruption or slowing of the infusion, as well as management of symptoms. Permanent discontinuation of daratumumab was to be undertaken if three grade 3 or greater IRRs occurred.
The criteria for a dose delay were grade 4 hematologic toxicities, grade 3 thrombocytopenia with bleeding, febrile neutropenia, neutropenia with infection, or grade ≥3 nonhematologic toxicities. Administration of daratumumab was restarted upon recovery from toxicity to grade 2 or baseline, with the exception of grade 2 laryngeal edema or bronchospasm that required full recovery. If >2 consecutive planned doses of daratumumab were missed due to adverse events, treatment was to be permanently discontinued. No dose adjustments were required for patients with preexisting renal dysfunction.
Toxicity and response criteria
The National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE), version 4.03, was used to grade adverse events as well as to assign perceived attribution of these events to the study treatment regimen. By these criteria, toxicity was defined as an adverse event considered to be possibly, probably, or definitely related to treatment.
The response criteria for hematologic and clinical/organ response were defined by consensus opinion from the International Society of Amyloidosis.1 A hematologic complete response (CR) was defined as the absence of a monoclonal protein in serum and urine by immunofixation electrophoresis, and a normal immunoglobulin free light chain ratio. A modified hematologic complete response (mCR) was defined as the absence of monoclonal protein in serum and urine by immunofixation electrophoresis, and a normal involved immunoglobulin free light chain concentration. Minimal residual disease (MRD) evaluation was performed by a validated multiparameter flow method using 10-color combinations with surface antigens for the identification of phenotypically aberrant clonal plasma cells in the bone marrow aspirate at the completion of protocol-directed treatment. At least 2 × 106 events were measured using a Beckman Coulter Navios flow cytometer and analyzed using Kaluza software. MRD positivity was defined as a phenotypically abnormal plasma cell population comprising ≥20 events with ≥2 aberrancies (minimum sensitivity, 1 in 105 nucleated cells).15 Organ responses to treatment were defined based on consensus guidelines and were based on biomarkers.16,17 Hematologic responses with serum free light chain levels were assessed every week for the first 2 cycles and with full comprehensive assessment along with serum and urine immunofixation electrophoreses, which were assessed at every 3 months. Organ responses were assessed every 3 months while on study treatment. Progression was defined as time to next line of treatment or death.
Statistical design and analysis
The study was designed as a single-arm phase 2 trial, in which the response in 20 evaluable patients was to be determined, with 25 patients enrolled to ensure that 20 were evaluable. An interim analysis was planned after the first 5 patients had completed at least 3 doses of daratumumab. The recommendation was to consider stopping the study for safety concerns if, during this time, 3 or more grade ≥4 adverse events clearly related to study drug were recorded. At this time, if 3 or more of the first 5 patients experienced a grade 3 infusion reaction clearly related to study drug, the protocol would be reevaluated to consider modifying the schedule of daratumumab (for example, splitting the first dose of daratumumab over 2 days) or adding mandatory concomitant medications. If <3 of the first 5 patients experienced a grade 3 infusion reaction, the study would reopen and enrollment would be expanded to an additional cohort of subjects.
Statistical analysis was based on all eligible subjects. The safety analysis population included all enrolled subjects who received at least 1 dose of study drug. The safety analysis population was used for evaluating subject characteristics, treatment administration, safety end points, and efficacy analyses. Descriptive summary statistics were used. Data were summarized primarily by administration time and aggregated across the first 8 weeks. Continuous variables were summarized by the number of nonmissing observations, mean, standard deviation, median, minimum, and maximum. Categorical variables were summarized by the number of nonmissing observations, frequencies, and percentages. All analyses were performed using SAS (SAS Institute Inc., Cary, NC) version 9.4 or higher.
Results
Patient characteristics
From April 2017 to August 2018, 25 patients with AL amyloidosis were screened for the trial. There were 22 patients eligible to receive treatment and 3 screen failures due to comorbid conditions, patient choice, or bone marrow plasmacytosis of >30%. The median age of enrolled patients was 65 years (range, 42-84 years); 16 men and 6 women enrolled. The majority of the patients (77%; n = 17) had λ clonal plasma cell dyscrasia. The distribution of organ involvement was typical for AL amyloidosis. Renal involvement was present in 15 patients (68%) and cardiac involvement in 14 patients (64%). Six patients (27%) had isolated renal involvement, and 4 (18%) had >2 organ systems involved. Three patients at the time of enrollment had abnormal renal function, with a serum creatinine level of ≥2.0 mg/dL. The median eGFR as determined by the Chronic Kidney Disease Epidemiology Collaboration equation (CKD-EPI) was 58 mL/min/1.73 m2 (range, 20-112 mL/min/1.73 m2). The renal stages were stage I in 11 patients, stage II in 10 patients, and stage III in 1 patient. Twenty patients (91%) had cardiac biomarker stage II or III disease. The median concentration of NT-proBNP was 1264 pg/mL (range, 32-3962 pg/mL) and of troponin I was 0.0345 ng/mL (range, <0.006-0.292 ng/mL), the median urine protein excretion was 0.53 g/24 hours (range, 0-10.1 g/24 hours), and the median difference in involved and uninvolved light chain levels (dFLC) was 80.7 mg/L (range, 2.9-854 mg/L). Seven patients had a dFLC < 50 mg/L. All patients had received prior therapies with a median of 2 prior therapies (range, 1-6). The majority of the patients had received HDM/SCT and/or proteasome inhibitor–based therapies. The median time following last plasma cell–directed treatment prior to enrollment on the trial was 9 months (range, 1-180 months). Twelve of 22 patients were refractory to the prior line of therapy. Of note, 3 patients (14%) had undergone prior solid organ transplantation (renal transplantation, n = 2; heart transplantation, n = 1). The median time from solid organ transplantation to enrollment on this trial was 7.4 years (range, 2.1-8.4 years) (Table 1).
Patient characteristics
| Data cutoff 1 August 2019 . | N = 22 . |
|---|---|
| Median age (range), y | 63 (42-83) |
| Median time since initial diagnosis (range), mo | 48 (8-184) |
| Median no. of organ systems involved, n (%) | 2 (1-5) |
| Cardiac biomarker stage II or III disease, n (%) | 20 (91) |
| NYHA class II or III, n (%) | 11 (50) |
| Median NTproBNP (range), pg/mL | 1264 (32-3962) |
| Median troponin I (range), ng/mL | 0.0345 (<0.006-0.292) |
| Median urine protein excretion (range), g/24 h | 0.53 (0-10.1) |
| Median eGFR (range), mL/min/1.73 m2 | 58 (20-112) |
| Renal stage II or III disease, n (%) | 11 (50) |
| Median no. of BM plasmacytosis (range), % | 10 (5-20) |
| Median time since last plasma cell–directed treatment (range), mo | 9 (1-180) |
| Median no. of prior therapies (range) | 2 (1-7) |
| High-dose melphalan/SCT, n (%) | 15 (68) |
| Proteasome inhibitor, n (%) | 16 (73) |
| Immunomodulatory agent, n (%) | 9 (41) |
| Median dFLC (range), mg/L | 80.7 (2.9-854) |
| Data cutoff 1 August 2019 . | N = 22 . |
|---|---|
| Median age (range), y | 63 (42-83) |
| Median time since initial diagnosis (range), mo | 48 (8-184) |
| Median no. of organ systems involved, n (%) | 2 (1-5) |
| Cardiac biomarker stage II or III disease, n (%) | 20 (91) |
| NYHA class II or III, n (%) | 11 (50) |
| Median NTproBNP (range), pg/mL | 1264 (32-3962) |
| Median troponin I (range), ng/mL | 0.0345 (<0.006-0.292) |
| Median urine protein excretion (range), g/24 h | 0.53 (0-10.1) |
| Median eGFR (range), mL/min/1.73 m2 | 58 (20-112) |
| Renal stage II or III disease, n (%) | 11 (50) |
| Median no. of BM plasmacytosis (range), % | 10 (5-20) |
| Median time since last plasma cell–directed treatment (range), mo | 9 (1-180) |
| Median no. of prior therapies (range) | 2 (1-7) |
| High-dose melphalan/SCT, n (%) | 15 (68) |
| Proteasome inhibitor, n (%) | 16 (73) |
| Immunomodulatory agent, n (%) | 9 (41) |
| Median dFLC (range), mg/L | 80.7 (2.9-854) |
BM, bone marrow; NYHA, New York Heart Association; SCT, stem cell transplantation.
Treatment experience
Of the 22 patients enrolled, 7 patients continue on protocol-directed therapy at the data cutoff of 1 August 2019. Nine patients have completed 24 months of protocol-directed therapy, 3 were removed from the protocol due to progression of plasma cell dyscrasia, 2 were removed after 8 cycles due to patient choice, and 1 was removed due to the persistent grade 3 adverse event of muscle weakness. A total of 594 infusions have been completed. The median number of infusions received per patient is 31 (range, 7-34). The median time of first infusion was 7 hours and second infusion was 4 hours 30 minutes.
Treatment-related adverse events
No patient experienced a grade 3/4 IRR. Four patients (19%) experienced grade 1 nausea and vomiting during first infusion, which resolved after an antiemetic. No other patient experienced nausea/vomiting after introduction of ondansetron in preinfusion medications. One patient experienced grade 1 “itchy throat” during first infusion that did not require any intervention. There were no hospitalizations for IRR. Grade 3 and 4 adverse events were noted in 20 patients (91%) (Table 2). Respiratory illnesses occurred in 13 patients (59%), 4 of whom (18%) had grade 3 (pneumonia, influenza A, rhino/enterovirus, Pneumocystis jiroveci pneumonia) infections. Three of these 4 patients with grade 3 infections had an immunoglobulin G level of <400 mg/dL at the time of the event. Atrial fibrillation and congestive heart failure (CHF), grade 3 and 4, occurred in 18% and 14% of patients, respectively. Iron deficiency requiring parenteral iron infusions or oral supplementation occurred in 9 patients (40%). Median time to onset of iron deficiency from enrollment was 5.6 months (range, 0.9-13.9 months). None of these patients had ferritin < 20 ng/mL. All patients had iron saturation of <20%. Two patients had Hgb < 10 g/dL but normal or elevated ferritin, and 1 patient had normal Hgb concentration with ferritin of <20 ng/mL. None of these patients requiring parenteral iron infusions had any overt bleeding, although 5 of 9 patients were on anticoagulation.
Treatment-related adverse events
| N = 22 . | Grades 3 and 4, N (%) . |
|---|---|
| Fatigue | 2 (9) |
| Respiratory infections | 4 (18) |
| Gastrointestinal bleeding | 1 (5) |
| Increased creatinine | 1 (5) |
| Atrial fibrillation | 4 (18) |
| Congestive heart failure | 3 (14) |
| Lymphopenia | 3 (14) |
| Diarrhea | 2 (9) |
| N = 22 . | Grades 3 and 4, N (%) . |
|---|---|
| Fatigue | 2 (9) |
| Respiratory infections | 4 (18) |
| Gastrointestinal bleeding | 1 (5) |
| Increased creatinine | 1 (5) |
| Atrial fibrillation | 4 (18) |
| Congestive heart failure | 3 (14) |
| Lymphopenia | 3 (14) |
| Diarrhea | 2 (9) |
Hematologic responses
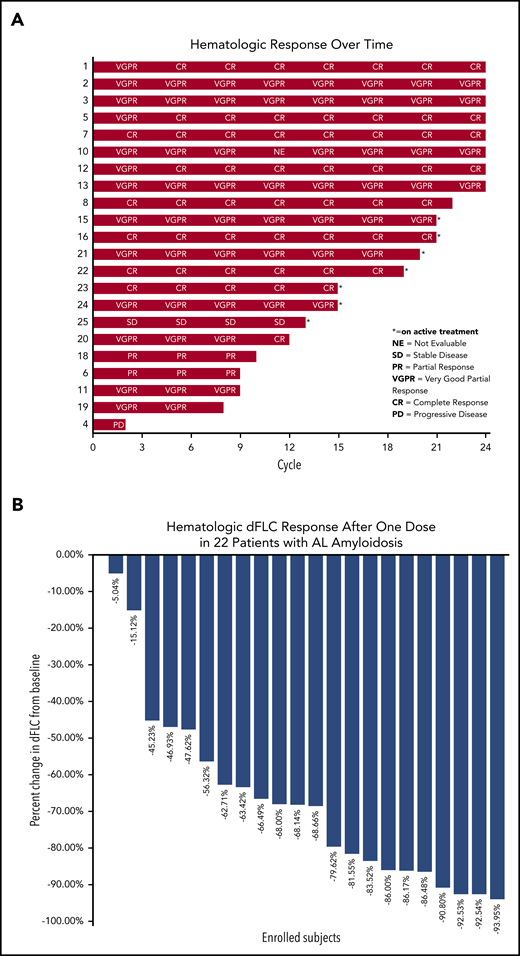
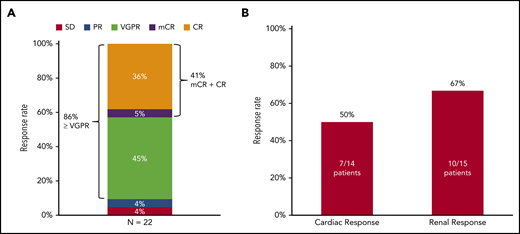
The median percentage of reduction in involved and uninvolved free light chain (dFLC) after 1 dose of daratumumab was 68.3% (Figure 1B). Hematologic CR was achieved by 9 patients (41%), very-good-partial response (VGPR) by 10 patients (45%), partial response (PR) by 1 patient (4%), and stable disease by 1 patient (4%) on an intention-to-treat analysis (Figures 1A and 2). One patient had progression of plasma cell dyscrasia markers during the first cycle and was removed from the protocol. Two patients, 1 with a PR after 3 cycles and 1 with VGPR after 4 cycles progressed subsequently at 9 and 10 cycles, respectively. Of the 9 patients with CR, 1 was graded as CR with mCR criteria (abnormal serum free light chain ratio but with normal involved free light-chain level). The median time to first free light chain level normalization/improvement-based hematologic response was 4 weeks (range, 1-51 weeks) and the mean time to first hematologic response was 7.64 weeks. The best hematologic response occurred at a median of 3 months (range, 3-12 months).
Hematologic response analysis by subject number over time. (A) The number of cycles of protocol treatment is shown on the x-axis and the subject number is shown on the y-axis; hematologic response analysis is shown on individual subject bars at the end. (B) Hematologic dFLC response after 1 dose of daratumumab in relapsed AL amyloidosis.
Hematologic response analysis by subject number over time. (A) The number of cycles of protocol treatment is shown on the x-axis and the subject number is shown on the y-axis; hematologic response analysis is shown on individual subject bars at the end. (B) Hematologic dFLC response after 1 dose of daratumumab in relapsed AL amyloidosis.
Hematologic and organ response. (A) Hematologic response by an intention-to-treat analysis. (B) Organ response.
Hematologic and organ response. (A) Hematologic response by an intention-to-treat analysis. (B) Organ response.
MRD
As of data cutoff on 1 August 2019, 14 of the 22 enrolled patients had end-of-treatment (EOT) visits. Ten of the 14 patients with EOT visits had undergone evaluation of the bone marrow for MRD assessment. Multiparametric flow cytometry (2-tube 10-color combination) identified MRD positivity at a minimum sensitivity of 10−5 in 7 patients (70%) and MRD negativity in 3 patients (30%). Of the 6 patients with hematologic CR, 3 were MRD− and 3 were MRD+. Of interest, the patient with achievement of mCR was MRD− at the EOT visit. A median of 54 monotypic plasma cells with abnormal phenotype (range, 23-156), corresponding to 0.004% plasma cells (range, 0.002% to 0.064%), were detected in patients with MRD positivity.
Organ responses
Organ response assessment was available in 21 patients, as 1 patient did not receive 3 cycles of treatment and organ response assessment was not performed. Renal response occurred in 10 of 15 patients (67%) with renal involvement and cardiac response occurred in 7 of 14 patients (50%) with cardiac involvement (Figure 2B). Renal progression was not noted in any of the patients, however, cardiac progression occurred in 3 patients (21%). The median time to first renal response was 18 weeks (range, 12-48 weeks) and first cardiac response was 20 weeks (range, 4-32 weeks). The median time to best renal response was 54 weeks (range, 12-88 weeks) and best cardiac response was 44 weeks (range, 16-92 weeks).
Time to next treatment
Three of 22 patients (14%) have received additional plasma cell–directed therapy at a median of 3 months (range, 1-6 months) after completion of protocol treatment. The median number of cycles of daratumumab administered in these 3 patients was 8 (range, 2-24). Of these, 2 patients have been treated with isatuximab on a clinical trial (clinicaltrials.gov identifier NCT03499808) and 1 patient with HDM/SCT. Only 1 patient of 3 had progressed while receiving daratumumab therapy; the patient then received HDM/SCT.
Deaths
Of the 22 patients enrolled, there were 3 deaths. All 3 patients were off clinical trial at the time of death. Two patients were removed from the clinical trial due to progression of plasma cell dyscrasia markers after 9 and 10 cycles of daratumumab, respectively. These 2 patients died at 9 and 4 months after completion of daratumumab, respectively. One patient died of a sudden death 3 months following completion of 24 cycles after having achieved a complete hematologic response. The causes of death were sepsis, immunomodulatory agent–related rejection of the transplanted heart (pomalidomide was being used for treatment of progressive plasma cell dyscrasia markers), and cardiac arrhythmia.
Progression-free survival
Median progression-free survival was 28 months with a median follow-up of surviving patients of 20 months (range, 11.9-27.6 months).
Discussion
In summary, daratumumab is well tolerated without grade 3 and 4 IRRs in patients with relapsed AL amyloidosis when used with appropriate preinfusion and postinfusion medications. It leads to rapid reduction in serum free light chain levels after 1 dose of daratumumab Figure 1B and the median time to first and best hematologic response is 4 weeks and 3 months, respectively, with a high hematologic overall response (CR plus VGPR) rate of 86%. This hematologic response also translated into high organ response rates.
In clinical trials (monotherapy and combination treatments; N = 1530) of multiple myeloma with daratumumab, the incidence of any grade infusion reactions was 40% with the first infusion, 2% with second infusion, and cumulatively 4% with subsequent infusions.18 The incidence of infusion modification due to reactions was 37%. However, in our current clinical trial of relapsed AL amyloidosis, IRRs were only mild, and serious grade 3 and 4 IRRs were not noted. We attribute this to utilization of a rigorous preinfusion and postinfusion medication schedule. Of note, appropriate medications were also administered 2 hours after start of infusion during the first 2 infusions even if there was no reaction. Prevention of IRRs is crucial in patients with AL amyloidosis as occurrence of IRR could be detrimental to the outcome of patients with multiorgan and cardiac involvement. Other recent retrospective studies report a grade 1 or 2 IRR in 22% to 60% of patients with relapsed AL amyloidosis.11,19
Serious adverse events of grade 3 and 4 with respect to atrial fibrillation and CHF occurred in 18% and 14% of patients, respectively, on this trial. This is much higher than what is reported in the experience of daratumumab in myeloma, CASTOR trial reported 2% patients experiencing atrial fibrillation in DVd arm (daratumumab, bortezomib, and dexamethasone)20 and CHF has not been reported in myeloma patients receiving daratumumab. These adverse events may be not related to daratumumab but related to the nature of AL amyloidosis and organ involvement. Additionally, CHF and fluid overload could be related to IV administration of daratumumab and the volume of normal saline that it is formulated in specifically with cardiac involvement. This adverse event may be ameliorated with subcutaneous formulation of daratumumab.21 We did identify an unusual occurrence of iron deficiency requiring parenteral iron infusions in 36% of the patients without significant anemia or bleeding and the etiology of this is unclear. Interestingly, anemia has been reported in myeloma in 45% of cases,22 of which 19% are grade 3. Iron studies should potentially be checked in patients with multiple myeloma who develop anemia while receiving daratumumab. Respiratory tract infections of grade 3 and 4 were observed in 18% of patients in the current study, however, these are reported only in 3% to 6% of patients with myeloma, which is a bit surprising as patients with myeloma are more immunocompromised and prone to infections.23
Hematologic responses achieved with single-agent daratumumab are rapid and median time to best hematologic response is 4 weeks. This is similar to other reports in AL amyloidosis. We hypothesize that because the burden of plasma cells in AL amyloidosis is low, hematologic responses are rapid and high in the range of 90% (CR plus VGPR) even with single-agent daratumumab. However, 50% of patients with hematologic CR are MRD+ by next-generation flow cytometry. The clinical implication of this is not yet known.
Organ responses were also seen in high numbers of patients on this trial. We attribute this to deep hematologic responses. The kinetics of organ responses are interesting with the median time of best cardiac response and best renal response being 11 and 13.5 months, respectively. This is again similar to median time to organ response from the start of treatment of 10.4 months (range, 8.7-12.8 months).24 It is a reminder that organ responses can be delayed after achievement of a hematologic response, and, hence, additional treatment or changes in treatment should not be considered due to lack of organ response in the first 6 to 12 months, as long as hematologic responses are favorable.24,25
A unique group of patients with relapsed AL amyloidosis after a solid organ transplantation were treated on this trial. There was no rejection of the transplanted organ or need for adjustment of immunosuppressive medications while on daratumumab. This is an important point as treatment choices are limited for relapsed AL amyloidosis after solid organ transplantation due to previously described organ rejection associated with immunomodulatory agents.26
The optimal duration of daratumumab treatment in patients with relapsed AL amyloidosis is unclear. Our trial arbitrarily incorporated 24 cycles of daratumumab and one could argue that the duration of treatment should be shortened as hematologic responses are rapid. Additionally, we do not have any prospective clinical trials comparing daratumumab monotherapy to daratumumab combined therapy (either with proteasome inhibitors or immunomodulatory agents) in patients with AL amyloidosis. A clinical trial of daratumumab in combination with cyclophosphamide, bortezomib, and dexamethasone for newly diagnosed patients with AL amyloidosis has completed accrual, and the results are anticipated (clinicaltrials.gov identifier: NCT03201965).
In summary, daratumumab appears safe and tolerable in patients with relapsed AL amyloidosis. It also is highly effective in inducing hematologic and organ responses. Notably, these results are more robust than achieved in myeloma, highlighting its presence in the therapeutic armamentarium of AL amyloidosis. In addition, a favorable toxicity profile makes daratumumab an attractive treatment option for patients with advanced cardiac involvement with AL amyloidosis. Extended follow-up will be needed to outline overall survival and, more importantly, progression-free survival.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgment
This work was partly supported by Janssen Research and Development, which provided daratumumab.
Authorship
Contribution: V.S. designed the study, performed research, analyzed the data, performed statistical analysis, and wrote the manuscript; S.S., J.M.S., and D.B. enrolled and evaluated subjects on clinical trial, edited the manuscript, and approved the final version of the manuscript; and A.S., M.M., M.E.M., R.C., and A.C.S. collected data, provided adverse event assessments, analyzed data, edited the manuscript, and approved the final version of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Vaishali Sanchorawala, Section of Hematology and Oncology, Boston Medical Center, 820 Harrison Ave, FGH 1007, Boston, MA 02118; e-mail: vaishali.sanchorawala@bmc.org.