Key Points
Presenting features plus degree of response, assessed by a simple flow assay, identify children with ALL at VLR of relapse.
Substantially reducing treatment intensity does not adversely affect the outcomes of a VLR ALL subset.
Abstract
Treatment-related mortality is common among children with acute lymphoblastic leukemia (ALL) treated in poor-resource settings. We applied a simplified flow cytometric assay to identify patients with precursor B-cell ALL (B-ALL) at very low risk (VLR) of relapse and treated them with a reduced-intensity treatment plan (RELLA05). VLR criteria include favorable presenting features (age ≥ 1 and < 10 years), white blood cell count of <50 ×109/L, lack of extramedullary leukemia, and minimal residual disease level of <0.01% on remission induction day 19. Except for 2 doses of daunorubicin, treatment of patients with VLR B-ALL consisted of a combination of agents with relatively low myelotoxicity profiles, including corticosteroids, vincristine, L-asparaginase, methotrexate, and 6-mercaptopurine. Cyclophosphamide, systemic cytarabine, and central nervous system radiotherapy were not used. Of 454 patients with ALL treated at the Instituto de Medicina Integral Professor Fernando Figueira in Recife, Brazil, between December 2005 and June 2015, 101 were classified as having VLR B-ALL. There were no cases of death resulting from toxicity or treatment abandonment during remission induction. At a median follow-up of 6.6 years, there were 8 major adverse events: 6 relapses, 1 treatment-related death (from septicemia) during remission, and 1 secondary myeloid leukemia. The estimated 5-year event-free and overall survival rates were 92.0% ± 3.9% and 96.0% ± 2.8%, respectively. The 5-year cumulative risk of relapse was 4.24% ± 2.0%. The treatment was well tolerated. Episodes of neutropenia were of short duration. Patients with B-ALL selected by a combination of presenting features and degree of early response can be successfully treated with a mildly myelosuppressive chemotherapy regimen.
Introduction
Risk-adapted intensification of chemotherapy coupled with improvements in supportive care1-3 has resulted in overall survival (OS) rates exceeding 90% for children with precursor B-cell acute lymphoblastic leukemia (B-ALL).4-6 However, the associated acute and chronic health disabilities have dimmed this success.7 Historically, 30% of children with ALL were cured with much less intensive regimens,8 but the lack of precise criteria by which to identify patients who could be cured with reduced-intensity regimens has hindered progress in decreasing treatment intensity. In limited-resource countries, attempts to increase treatment intensity for children with ALL have not translated into improved outcomes.9,10 Socioeconomic and cultural barriers and a lack of hospital infrastructure have resulted in high rates of treatment-related mortality and treatment abandonment.11 In this context, there is a strong rationale for identifying a subset of ALL patients who can be treated with a reduced-intensity regimen.12
In a previous study of children with ALL treated with standard-intensity regimens, those patients with a minimal residual disease (MRD) level of <0.01% early in remission induction had very low relapse rates.13 These findings led us to propose that children with ALL with favorable presenting features and a major early response to induction therapy (MRD <0.01%) could be selected to receive reduced–dose intensity treatment regimens. We hypothesized that patients with highly drug-sensitive ALL treated with a low-intensity regimen would have low rates of death from toxicity, resulting in high rates of disease-free survival. Here, we report treatment outcomes for 101 of 375 consecutive patients with very-low-risk (VLR) B-ALL who were treated with an antimetabolite-based regimen in a low-resource center in Recife, Brazil.
Patients and methods
Patients
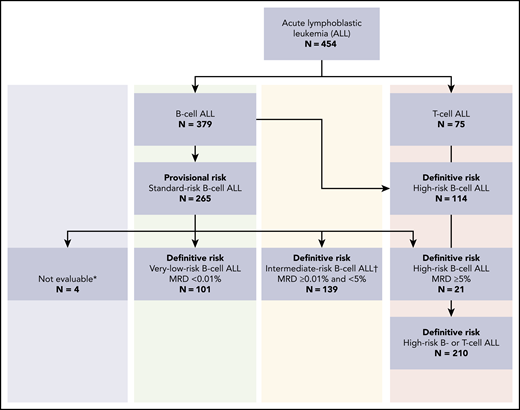
From December 2005 to June 2015, a total of 454 consecutive patients (age 1 to <18 years) with newly diagnosed ALL were treated at the Instituto de Medicina Integral Professor Fernando Figueira (IMIP), using the uniform guidelines of the Recife ALL pilot study14 (RELLA05). Of the 379 children with B-ALL, 375 were evaluated for MRD on remission induction day 19. Of these, 101 (26.9%) exhibited initial features associated with low-risk ALL1 and MRD of <0.01% on days 19 and 26 (Figure 1). The IMIP institutional review board approved the treatment plan. Parents/legal guardians or patients signed written, informed consent and assent, respectively.
Provisional and definitive risk classification of 454 patients with acute lymphoblastic leukemia registered in the RELLA05 protocol. *Four patients did not have procedures on day 19. †MRD analysis was inconclusive in 14 patients with provisional standard risk; they were treated in the intermediate-risk group.
Provisional and definitive risk classification of 454 patients with acute lymphoblastic leukemia registered in the RELLA05 protocol. *Four patients did not have procedures on day 19. †MRD analysis was inconclusive in 14 patients with provisional standard risk; they were treated in the intermediate-risk group.
Diagnosis and risk classification
Diagnostic criteria for ALL were based on morphologic, immunophenotypic, and genetic analyses of bone marrow cells, performed using standard techniques.1 The classification of central nervous system (CNS) involvement (CNS status 1, 2, or 3 or traumatic tap) has been described previously.15,16
ALL risk classification was based on clinical and biologic features. Patients with B-ALL who were age ≥1 but <10 years and had a white blood cell (WBC) count of <50 ×109/L, along with those with a WBC count of ≥50 ×109/L but a DNA index of ≥1.16 or ETV6-RUNX1 rearrangement, were classified as having provisional low-risk ALL. Patients with BCR-ABL1, TCF3-PBX1, KTM2A-AFF1, or KTM2A-MMLT1 rearrangements; those with extramedullary disease (CNS or testicular involvement); and those age <1 or ≥10 years were considered to have high-risk ALL. The definitive risk classification was determined according to the MRD level in the bone marrow. Patients with provisional low-risk ALL and MRD of <0.01% on days 19 and 26 of remission induction were considered to have VLR B-ALL. Patients with provisional low-risk ALL and MRD of ≥0.01% but <5% on day 19 of remission induction were considered to have intermediate-risk ALL, whereas those with MRD of ≥5% were considered to have high-risk ALL (Figure 1; Table 1).
Risk classification of ALL (RELLA05)
| Feature . | VLR ALL (n = 101) . | Standard-risk ALL (n = 139) . | High-risk ALL (n = 210) . |
|---|---|---|---|
| Age, y | 1-9 | Any | Any |
| Lineage | B | B | B or T |
| WBC count, ×109/L | <50.0* | Any | Any |
| CNS status | 1, 2, traumatic | 1, 2, traumatic | 1, 2, 3, traumatic |
| Testicular involvement | None | None | Any |
| Skin involvement | None | None | Any |
| MRD d 19, % | <0.01 | 0.01 to <5 | Any |
| Feature . | VLR ALL (n = 101) . | Standard-risk ALL (n = 139) . | High-risk ALL (n = 210) . |
|---|---|---|---|
| Age, y | 1-9 | Any | Any |
| Lineage | B | B | B or T |
| WBC count, ×109/L | <50.0* | Any | Any |
| CNS status | 1, 2, traumatic | 1, 2, traumatic | 1, 2, 3, traumatic |
| Testicular involvement | None | None | Any |
| Skin involvement | None | None | Any |
| MRD d 19, % | <0.01 | 0.01 to <5 | Any |
Patients with ETV6-RUNX1 fusion transcripts or DNA index ≥1.16 were eligible for inclusion in the VLR ALL group regardless of WBC count at diagnosis.
Measurement of residual disease
MRD levels were measured as previously described,13 although the method was simplified to adapt it to local resources.17 Briefly, mononucleated cells were obtained by using Ficoll Histopaque. Antibodies were added as follows: tube 1, anti-human CD34 fluorescein isothiocyanate (FITC)/CD10 phycoerythrin (PE)/CD19 PerCP-Cy5.5 (BD Biosciences, San Jose, CA); control tube 2, anti-human CD34 FITC/immunoglobulin G1 PE/CD19 PerCP-Cy5.5 (BD Biosciences); and tube 3, anti-human κ/Λ FITC/CD10 PE/CD19 PerCP-Cy5.5 (BD Biosciences). After a 10-minute incubation in the dark, cells were washed twice with phosphate-buffered saline A and fixed with 0.5% paraformaldehyde. An additional aliquot of cells was stained with Syto13 (Thermo Fisher Scientific, Waltham, MA) and with anti-human CD19 PerCP-Cy5.5 as a correction factor to determine the viable nucleated cell count; these cells were not fixed. For the first 302 patients, antibody labeling was analyzed with a single-laser FACSCalibur flow cytometer (BD Biosciences) using CellQuest Pro software (version 8.0). For the remaining patients, MRD was quantified with a 2-laser Accuri flow cytometer (BD Biosciences) using the CFlow Plus software. In all cases, there were 2 acquisition steps: acquiring 10 000 events and acquiring as many events as possible. The total number of viable mononucleated cells acquired was at least 1 × 105, which is the minimum number required for a sensitivity of detection of 1 in 10 000. The MRD calculation was based on the percentage of viable CD19+ cells coexpressing CD10 and/or CD34 among the total acquired mononucleated cells. The MRD was considered positive for values of ≥0.01%. Flow cytometric data analysis of all samples obtained up to May 2010 was verified by 1of the authors (E.C.-S.); after that period, data analysis was performed by the local flow cytometric team.
Treatment
Table 2 depicts the chemotherapy schedule. For the first 4 days, chemotherapy consisted of prednisone alone to allow sufficient time to stabilize patients and correct metabolic and infectious complications. On day 5, remission induction was started with 1 dose of vincristine per week for 4 weeks, 1 dose of daunorubicin per week for 2 weeks, and 6 doses of (3 times a week) of L-asparaginase (ELSPAR asparaginase; Merck, Sharp & Dohme). Prednisone was administered daily for a total of 28 days. After hematopoietic recovery, patients in morphologic remission received consolidation with high-dose methotrexate (HD-MTX) and mercaptopurine. During the first 6 weeks of continuation therapy, patients received 1 course of dexamethasone and vincristine in the first week, followed by daily mercaptopurine and weekly parenteral MTX in the remaining 5 weeks. From week 7 through week 12, early intensification (reinduction) consisted of 1 dose of vincristine and asparaginase per week for 4 weeks, 2 weeks of dexamethasone (in weeks 7 and 9), 1 dose of HD-MTX (at the start of week 11), and daily doses of mercaptopurine for 14 days (weeks 11 and 12). From week 13 through week 52, patients received daily mercaptopurine, weekly parenteral MTX, and pulses of dexamethasone and vincristine every 4 weeks. For the remainder of continuation therapy (weeks 53 to 104), patients received mercaptopurine daily and parenteral MTX weekly.
Remission induction, consolidation, early continuation, reinduction therapy, and continuation A and B
| Agent . | Dosage . | Route and schedule . |
|---|---|---|
| Remission induction (4 wk) | ||
| Prednisone | 40 mg/m2/d | Orally d 1-28 |
| Vincristine | 1.5 mg/m2/wk | IV d 5, 12, 19, 26 |
| Daunomycin | 25 mg/m2/wk | IV d 5, 12 |
| L-asparaginase (Escherichia coli) | 10 000 U/m2 thrice weekly | IM d 6, 8, 10, 12, 14, 16 |
| Triple intrathecal | Age dependent1 | IT d 5, (12),* 19 |
| Consolidation (8 wk) | ||
| HD-MTX | 2.5 g/m2, 4-h infusion | IV d 1, 15, 29, 43 |
| Mercaptopurine | 50 mg/m2/d | IV d 1-56 |
| Triple intrathecal | Age dependent1 | IT d 1, 15, 29, 43 |
| Early continuation (6 wk) | ||
| Dexamethasone | 6 mg/m2/d | Orally d 1-7 |
| Vincristine | 2 mg/m2 | IV d 1 |
| Mercaptopurine | 75 mg/m2/d | Orally d 8-42 |
| MTX | 40 mg/m2/wk | IV/IM d 8, 15, 22, 29, 36 |
| Triple intrathecal | Age dependent1 | IT d 15 |
| Reinduction (6 wk) | ||
| Dexamethasone | 6 mg/m2/d | Orally d 1-7, 15-21 |
| Vincristine | 1.5 mg/m2/wk | IV d 1, 8, 15, 22 |
| L-asparaginase (E coli) | 25 000 U/m2/wk | IM d 1, 8, 15, 22 |
| HD-MTX | 2.5 g/m2, 4-h infusion | IV d 29 |
| Mercaptopurine | 50 mg/m2/d | Orally d 29-42 |
| Triple intrathecal | Age dependent1 | IT d 1, 29 |
| Continuation A (40 wk) | ||
| Mercaptopurine | 75 mg/m2/d × 7 d | Orally wk 25-64 |
| MTX | 40 mg/m2/wk | IV/IM wk 25, 26, 28-30, 32-34, 36-38, 40-42, 44-46, 48-50, 52-54, 56-58, 60-63 |
| Vincristine | 1.5 mg/m2/wk | IV wk 27, 31, 35, 39, 43, 47, 51, 55 |
| Dexamethasone | 6 mg/m2/d × 5 d | Orally wk 27, 31, 35, 39, 43, 47, 51, 55, 59, 64 |
| Triple intrathecal | Age dependent1 | IT wk 27, 35, 43, 51, 59, 64 |
| Continuation B (52 wk) | ||
| Mercaptopurine | 75 mg/m2/d × 7 d | Orally wk 65-116 |
| MTX | 40 mg/m2 | IV/IM wk 65-116 |
| Triple intrathecal | Age dependent1 | IT wk (72, 80, 88, 96, 104, 112)* |
| Agent . | Dosage . | Route and schedule . |
|---|---|---|
| Remission induction (4 wk) | ||
| Prednisone | 40 mg/m2/d | Orally d 1-28 |
| Vincristine | 1.5 mg/m2/wk | IV d 5, 12, 19, 26 |
| Daunomycin | 25 mg/m2/wk | IV d 5, 12 |
| L-asparaginase (Escherichia coli) | 10 000 U/m2 thrice weekly | IM d 6, 8, 10, 12, 14, 16 |
| Triple intrathecal | Age dependent1 | IT d 5, (12),* 19 |
| Consolidation (8 wk) | ||
| HD-MTX | 2.5 g/m2, 4-h infusion | IV d 1, 15, 29, 43 |
| Mercaptopurine | 50 mg/m2/d | IV d 1-56 |
| Triple intrathecal | Age dependent1 | IT d 1, 15, 29, 43 |
| Early continuation (6 wk) | ||
| Dexamethasone | 6 mg/m2/d | Orally d 1-7 |
| Vincristine | 2 mg/m2 | IV d 1 |
| Mercaptopurine | 75 mg/m2/d | Orally d 8-42 |
| MTX | 40 mg/m2/wk | IV/IM d 8, 15, 22, 29, 36 |
| Triple intrathecal | Age dependent1 | IT d 15 |
| Reinduction (6 wk) | ||
| Dexamethasone | 6 mg/m2/d | Orally d 1-7, 15-21 |
| Vincristine | 1.5 mg/m2/wk | IV d 1, 8, 15, 22 |
| L-asparaginase (E coli) | 25 000 U/m2/wk | IM d 1, 8, 15, 22 |
| HD-MTX | 2.5 g/m2, 4-h infusion | IV d 29 |
| Mercaptopurine | 50 mg/m2/d | Orally d 29-42 |
| Triple intrathecal | Age dependent1 | IT d 1, 29 |
| Continuation A (40 wk) | ||
| Mercaptopurine | 75 mg/m2/d × 7 d | Orally wk 25-64 |
| MTX | 40 mg/m2/wk | IV/IM wk 25, 26, 28-30, 32-34, 36-38, 40-42, 44-46, 48-50, 52-54, 56-58, 60-63 |
| Vincristine | 1.5 mg/m2/wk | IV wk 27, 31, 35, 39, 43, 47, 51, 55 |
| Dexamethasone | 6 mg/m2/d × 5 d | Orally wk 27, 31, 35, 39, 43, 47, 51, 55, 59, 64 |
| Triple intrathecal | Age dependent1 | IT wk 27, 35, 43, 51, 59, 64 |
| Continuation B (52 wk) | ||
| Mercaptopurine | 75 mg/m2/d × 7 d | Orally wk 65-116 |
| MTX | 40 mg/m2 | IV/IM wk 65-116 |
| Triple intrathecal | Age dependent1 | IT wk (72, 80, 88, 96, 104, 112)* |
IM, intramuscular; IT, intrathecal.
Patients with CNS status 2 or traumatic tap.
The CNS-directed therapy comprised intrathecal MTX, hydrocortisone, and cytarabine (ITMHA), followed 24 and 36 hours later by oral doses of leucovorin.1 In induction, 2 doses of ITMHA were administered to patients with CNS status 1, and 1 additional dose was administered on day 12 to those patients with CNS status 2 or traumatic tap, which was defined as having ≥10 red blood cells per microliter of cerebrospinal fluid (CSF). Four additional doses of ITMHA were administered biweekly during consolidation. One dose and 2 doses of ITMHA were administered in week 3 and in reinduction, respectively. For the remainder of the continuation therapy, patients with CNS status 1 received 6 doses of ITMHA (for a total of 15 doses) and those with traumatic tap received 12 doses (for a total of 22 doses). No patient received CNS irradiation.
The authors (F.P., M.M.L., R.C.R., G.K.R., and N.L.-S.) discussed all patients with newly diagnosed B-ALL and adverse events during treatment in weekly online meetings. The local physicians had the final decision on whether to implement recommended treatment interventions or modifications. During continuation chemotherapy, for patients with a WBC of <1.0 ×109/L or an absolute neutrophil count of <0.3 ×109/L, irrespective of the presence of fever, the protocol was paused for all chemotherapy agents, including vincristine and dexamethasone. Patients were reassessed after at least 48 hours, and chemotherapy was restarted if the patients were clinically well and signs of bone marrow recovery were noted. Chemotherapy was restarted from the time it was paused; thus, all pulses of vincristine and dexamethasone planned for continuation phases were administered.
Statistical analysis
Differences in the distribution of clinical and biologic variables between subsets of patients were analyzed by using the χ2 test or Fisher’s exact test for categorical variables and the Student t test for continuous variables. OS was defined as the time interval from the date on study to death resulting from any cause or to last follow-up. Event-free survival (EFS) was defined as the time interval from the date on study to any leukemia relapse, second malignancy, or death resulting from any cause. The Kaplan-Meier method was used to estimate OS and EFS. Time was censored at the last follow-up date if no failure was observed. The cumulative incidence of relapse from the complete remission date to any relapse was estimated using the Kalbfleisch-Prentice estimator. Second cancers or deaths resulting from any cause were regarded as competing events. Patients who remained alive and in remission were censored at the time of their last follow-up.
The database as of February 2019 was used for analysis. All patients with definitive low risk completed therapy, and all survivors were seen 1 to 4 times a year. All reported P values are 2 sided and were not adjusted for multiple tests.
Results
Table 3 summarizes the characteristics of 375 of 379 patients with B-ALL evaluated for MRD at days 19 and 26. The remaining 4 patients were not evaluable at day 19 of induction and were removed from the study. On the basis of MRD levels on days 19 and 26, 101 of 257 patients with provisional low-risk ALL were reclassified as having VLR ALL. Six patients with negative MRD on day 19 and MRD of ≥0.01% on day 26 were reclassified as having intermediate-risk ALL. The median age of the 53 boys and 48 girls with VLR ALL was 4.0 years (range, 1.2-9.8 years). The self-reported race was white for 31 patients (30.7%), black for 4 patients (4.0%), and mixed for 66 patients (65.3%). The median WBC count was 7.0 ×109/L (range, 1.0 ×109 to 89.9 ×109/L). The CNS was not involved (CNS status 1) in 91 patients (90.1%); in the remaining 10 patients, CSF examination showed >10 RBCs per mL (bloody/traumatic tap). The DNA index was available for 98 patients and was ≥1.16 for 29 (28.7%) and 1.0 for 69 (71.93%). A panel to detect 5 rearrangements (ETV6-RUNX1, TCF3-PBX1, BCR-ABL1, KMT2A-AFF1, and KTM2A-MMLT1) by polymerase chain reaction was performed for 97 patients. The ETV6-RUNX1 rearrangement was detected in 31 patients; the remaining 66 patients had none of the 4 rearrangements. In accordance with the National Cancer Institute risk classification,18 97 cases were classified as standard risk and 4 as high risk (cases with a WBC count of ≥50 ×109/L but with ETV6-RUNX1 rearrangement or a DNA index of ≥1.16). Compared with other patients with B-ALL, patients with VLR B-ALL were significantly more likely to have CNS 1 status, a lower WBC count at diagnosis, younger age, a DNA index of ≥1.16, ETV6-RUNX1 rearrangement, and higher rates of complete remission (Table 3).
Clinical and laboratory characteristics of patients with precursor B-ALL
| Characteristic . | Definitive risk classification, n (%)* . | P . | |
|---|---|---|---|
| Low risk (n = 101) . | Other (n = 274) . | ||
| Age, y | <.0001 | ||
| Median | 4.0 | 5.1 | |
| Range | 1.2-9.8 | 1.0-17.7 | |
| Sex | .7428 | ||
| Male | 53 (52.5) | 149 (54.4) | |
| Female | 48 (47.5) | 125 (45.6) | |
| Race | .1646 | ||
| Mixed | 66 (65.3) | 176 (64.2) | |
| White | 31 (30.7) | 95 (34.7) | |
| Black | 4 (4.0) | 3 (1.1) | |
| WBC count, ×109/L | .0011 | ||
| Median | 7.0 | 9.0 | |
| Range | 1.0-89.9 | 4.9-482.8 | |
| CNS | .0578 | ||
| Status 1 | 91 (90.1) | 240 (87.6) | |
| Status 2 | 0 | 2 (0.7) | |
| Status 3 | 0 | 13 (4.7) | |
| TP with blasts | 1 (1.0) | 7 (2.6) | |
| TP without blasts | 9 (8.9) | 12 (4.4) | |
| NCI risk | <.0001 | ||
| Standard | 97 (96.0) | 167 (60.9) | |
| High | 4 (4.0) | 107 (39.1) | |
| DNA index | .1459 | ||
| ≥1.16 | 29 (28.7) | 55 (20.1) | |
| Other | 69 (68.3) | 214 (78.1) | |
| No data | 3 (3.0) | 5 (1.8) | |
| Genotype | <.0001 | ||
| Negative | 66 (65.3) | 197 (71.9) | |
| ETV6-RUNX1 | 31 (30.7) | 29 (10.6) | |
| TCF3-PBX1 | 0 | 27 (9.9) | |
| BCR-ABL1 | 0 | 10 (3.6) | |
| KMT2A-AFF1 | 0 | 3 (1.1) | |
| KTM2A-MMLT1 | 0 (0) | 1 (0.4) | |
| No data | 4 (4.0) | 7 (2.6) | |
| MRD at d 19, % | <.0001 | ||
| <0.01 | 101 (100.0) | 49 (17.9) | |
| ≥0.01 | 0 | 211 (77.0) | |
| Inconclusive | 0 | 14 (5.1) | |
| Complete remission | .0652 | ||
| Yes | 101 (100.0) | 265 (96.7) | |
| No | 0 | 9 (3.3) | |
| Characteristic . | Definitive risk classification, n (%)* . | P . | |
|---|---|---|---|
| Low risk (n = 101) . | Other (n = 274) . | ||
| Age, y | <.0001 | ||
| Median | 4.0 | 5.1 | |
| Range | 1.2-9.8 | 1.0-17.7 | |
| Sex | .7428 | ||
| Male | 53 (52.5) | 149 (54.4) | |
| Female | 48 (47.5) | 125 (45.6) | |
| Race | .1646 | ||
| Mixed | 66 (65.3) | 176 (64.2) | |
| White | 31 (30.7) | 95 (34.7) | |
| Black | 4 (4.0) | 3 (1.1) | |
| WBC count, ×109/L | .0011 | ||
| Median | 7.0 | 9.0 | |
| Range | 1.0-89.9 | 4.9-482.8 | |
| CNS | .0578 | ||
| Status 1 | 91 (90.1) | 240 (87.6) | |
| Status 2 | 0 | 2 (0.7) | |
| Status 3 | 0 | 13 (4.7) | |
| TP with blasts | 1 (1.0) | 7 (2.6) | |
| TP without blasts | 9 (8.9) | 12 (4.4) | |
| NCI risk | <.0001 | ||
| Standard | 97 (96.0) | 167 (60.9) | |
| High | 4 (4.0) | 107 (39.1) | |
| DNA index | .1459 | ||
| ≥1.16 | 29 (28.7) | 55 (20.1) | |
| Other | 69 (68.3) | 214 (78.1) | |
| No data | 3 (3.0) | 5 (1.8) | |
| Genotype | <.0001 | ||
| Negative | 66 (65.3) | 197 (71.9) | |
| ETV6-RUNX1 | 31 (30.7) | 29 (10.6) | |
| TCF3-PBX1 | 0 | 27 (9.9) | |
| BCR-ABL1 | 0 | 10 (3.6) | |
| KMT2A-AFF1 | 0 | 3 (1.1) | |
| KTM2A-MMLT1 | 0 (0) | 1 (0.4) | |
| No data | 4 (4.0) | 7 (2.6) | |
| MRD at d 19, % | <.0001 | ||
| <0.01 | 101 (100.0) | 49 (17.9) | |
| ≥0.01 | 0 | 211 (77.0) | |
| Inconclusive | 0 | 14 (5.1) | |
| Complete remission | .0652 | ||
| Yes | 101 (100.0) | 265 (96.7) | |
| No | 0 | 9 (3.3) | |
NCI, National Cancer Institute.
Four patients were not evaluable for MRD on day 19.
Treatment compliance and toxicity
Treatment toxicity and compliance were evaluated for all 101 patients after they completed each treatment phase (Table 4). In induction, 85 (84.1%) of 101 patients received all planned therapy. Three patients missed 1 day each of the planned prednisone, and 1 of these 3 patients missed 1 dose of vincristine because of fever and neutropenia. Thirteen patients (12.8%) did not receive the second dose of daunorubicin because of fever or severe neutropenia. The 6 doses of L-asparaginase planned for the induction phase were administered to all patients. One patient developed an allergic reaction after the last dose of L-asparaginase. Hepatic and/or renal grade 3 or 4 toxicities occurred in 10 and 3 patients, respectively, but resolved without requiring specific interventions. The mean time between the start of the induction and consolidation courses was 30 days (range, 28-61 days). In consolidation, 94 (93.1%) of 101 patients received the 4 planned HD-MTX plus ITMHA courses. One patient received only 2 doses of HD-MTX plus ITMHA because of prolonged fever after the second dose. Grade 3 or 4 renal and/or hepatic toxicities occurred in 17 patients but resolved with supportive care measures. MTX plasma levels were not monitored. Except for 1 episode of hepatic grade 3 toxicity that resolved with supportive care measures, no nonhematologic toxicity was noted during this phase. In reinduction, 4 (3.9%) of 101 patients received only 3 of the 4 planned doses of vincristine because of fever and neutropenia. Hypersensitivity or allergic reaction to L-asparaginase was observed in 54 patients, although 98 (97%) of 101 patients received ≥80% of the planned doses (most patients developed allergic reactions after the eighth dose of L-asparaginase). Alternative preparations of L-asparaginase were not available. Treatment for weeks 13 through 104 (continuation A and B) was well tolerated. Hepatic toxicity was documented in 17 patients but resolved with supportive care measures. During all treatment phases, admissions for fever and neutropenia were common (n = 363). These episodes were usually uncomplicated and resolved within 3 to 5 days. Blood cultures were positive in 30 cases (8.2%; Table 4). Patients did not receive prophylactic antibiotics or antifungals. Death resulting from toxicity from infectious complications occurred in 1 patient in week 87 of continuation treatment. Symptomatic osteonecrosis, thromboembolic events, and severe pancreatitis were not reported in this cohort. The cumulative dose of selected chemotherapy agents administered according to the RELLA05 and St. Jude Total XV protocols is shown in supplemental Table 1. Other details of hematologic toxicity are shown in supplemental Table 2. As expected, hematologic toxicity of grade ≥3 was common but varied in frequency during the treatment phases. It occurred at least once in 96% of all patients during induction and in 65.3%, 14.8%, 53.4%, 68.3%, and 50% of patients during consolidation, early continuation, reinduction, and continuation A and B, respectively.
Grade ≥3 toxicity and treatment compliance (n = 101 patients)
| . | Induction 4 wk . | Consolidation 8 wk . | Early continuation 6 wk . | Reinduction 6 wk . | Continuation A 40 wk . | Continuation B 52 wk . |
|---|---|---|---|---|---|---|
| CNS toxicity, n of patients | 0 | 1 | 0 | 0 | 0 | 0 |
| Respiratory toxicity, n of patients | 0 | 0 | 0 | 0 | 0 | 0 |
| Hepatic toxicity, n of patients | 10 | 13 | 1 | 10 | 7 | 10 |
| Renal toxicity, n of patients | 3 | 4 | 0 | 4 | 0 | 1 |
| Hypersensitivity to L-asparaginase, n of patients | 1* | NA | NA | 53/100 | NA | NA |
| Neutropenia and fever, n of episodes (n of patients) | 61 (34) | 19 (10) | 20 (11) | 32 (19) | 115 (44) | 116 (54) |
| Blood cultures, n positive | 9 (5 gram+, 4 gram−) | 5 (2 gram+, 3 gram−) | None | 3 (3 gram+) | 5 (4 gram+, 1 gram−) | 8 (8 gram+) |
| Hospitalization, median d (range) | 3 (4-5) | 5 (2-15) | 4 (2-7) | 4 (2-19) | 2.5 (1-18) | 5 (1-22) |
| Deaths from toxicity, n | 0 | 0 | 0 | 0 | 0 | 1† |
| Received planned therapy, n of patients‡ | 85 | 94 | 101 | 47 | 101 | 100 |
| . | Induction 4 wk . | Consolidation 8 wk . | Early continuation 6 wk . | Reinduction 6 wk . | Continuation A 40 wk . | Continuation B 52 wk . |
|---|---|---|---|---|---|---|
| CNS toxicity, n of patients | 0 | 1 | 0 | 0 | 0 | 0 |
| Respiratory toxicity, n of patients | 0 | 0 | 0 | 0 | 0 | 0 |
| Hepatic toxicity, n of patients | 10 | 13 | 1 | 10 | 7 | 10 |
| Renal toxicity, n of patients | 3 | 4 | 0 | 4 | 0 | 1 |
| Hypersensitivity to L-asparaginase, n of patients | 1* | NA | NA | 53/100 | NA | NA |
| Neutropenia and fever, n of episodes (n of patients) | 61 (34) | 19 (10) | 20 (11) | 32 (19) | 115 (44) | 116 (54) |
| Blood cultures, n positive | 9 (5 gram+, 4 gram−) | 5 (2 gram+, 3 gram−) | None | 3 (3 gram+) | 5 (4 gram+, 1 gram−) | 8 (8 gram+) |
| Hospitalization, median d (range) | 3 (4-5) | 5 (2-15) | 4 (2-7) | 4 (2-19) | 2.5 (1-18) | 5 (1-22) |
| Deaths from toxicity, n | 0 | 0 | 0 | 0 | 0 | 1† |
| Received planned therapy, n of patients‡ | 85 | 94 | 101 | 47 | 101 | 100 |
NA, not applicable.
Patient did not receive L-asparaginase in reinduction.
Died as a result of infectious complications.
During episodes of fever and neutropenia in continuation, the protocol was paused.
Survival analysis
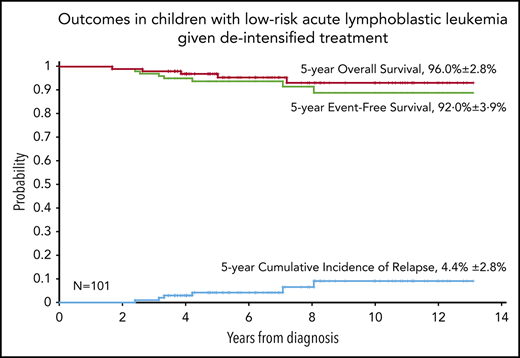
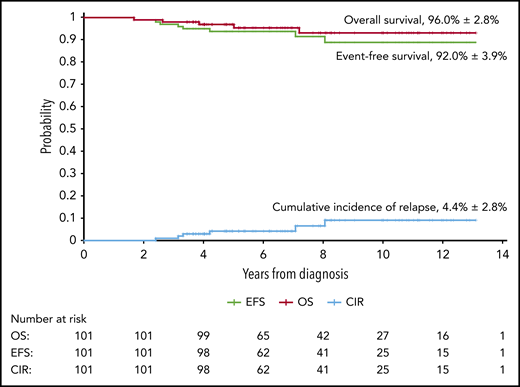
At a median follow-up of 6.6 years (range, 3.4-13.1 years), 5 boys and 1 girl had experienced relapse: 3 hematologic relapses, 1 combined CNS and hematologic relapse, 1 combined testicular and hematologic relapse, and 1 combined lymph node and kidney relapse. Of these 6 patients, 3 were alive in second remission. The remaining 3 patients who experienced relapse died as a result of complications associated with salvage therapy. One patient developed secondary acute myeloid leukemia after 4 months of pancytopenia and died as a result of treatment-related complications. As mentioned previously, 1 patient died in complete remission as a result of infectious complications. Table 5 summarizes the clinical characteristics and outcomes of 8 patients who experienced adverse events. The estimated 5-year EFS for 101 children with VLR ALL was 92.0% ± 3.9%, and the OS was 96.0% ± 2.8%. The 5-year cumulative risk of relapse was 4.4% ± 2.8% (Figure 2).
Clinical, laboratory, treatment, and outcome features of selected children who experienced relapse
| Patient n . | Age, y . | Sex . | Race . | WBC, ×109/L . | Genotype . | Relapse site . | HD-MTX doses received, % . | L-asparaginase doses received, % . | Duration of first CR, mo . | Outcome after salvage therapy . |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2.7 | Male | White | 8.6 | ETV6-RUNXT1 | Bone marrow | 100 | 80 | 37 | Died, infectious complications |
| 27 | 3.9 | Female | Mixed | 4.1 | ETV6-RUNXT1 | Lymph nodes and kidneys | 60 | 90 | 96 | Alive, second CR, 28 mo |
| 85 | 6.3 | Male | Mixed | 18.0 | DNA index 1 | Bone marrow and testicles | 100 | 100 | 39 | Alive, second CR, 12 mo |
| 324 | 3.9 | Male | White | 6.8 | DNA index 1 | Bone marrow | 100 | 80 | 27 | Died, infectious complications |
| 349 | 3.7 | Male | Mixed | 6.7 | DNA index 1 | Bone marrow | 100 | 100 | 28 | Died, infections complications |
| 363 | 2.6 | Male | Mixed | 14.1 | DNA index 1.16 | Bone marrow and CNS | 100 | 80 | 21 | Alive, second CR, 12 mo |
| Patient n . | Age, y . | Sex . | Race . | WBC, ×109/L . | Genotype . | Relapse site . | HD-MTX doses received, % . | L-asparaginase doses received, % . | Duration of first CR, mo . | Outcome after salvage therapy . |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2.7 | Male | White | 8.6 | ETV6-RUNXT1 | Bone marrow | 100 | 80 | 37 | Died, infectious complications |
| 27 | 3.9 | Female | Mixed | 4.1 | ETV6-RUNXT1 | Lymph nodes and kidneys | 60 | 90 | 96 | Alive, second CR, 28 mo |
| 85 | 6.3 | Male | Mixed | 18.0 | DNA index 1 | Bone marrow and testicles | 100 | 100 | 39 | Alive, second CR, 12 mo |
| 324 | 3.9 | Male | White | 6.8 | DNA index 1 | Bone marrow | 100 | 80 | 27 | Died, infectious complications |
| 349 | 3.7 | Male | Mixed | 6.7 | DNA index 1 | Bone marrow | 100 | 100 | 28 | Died, infections complications |
| 363 | 2.6 | Male | Mixed | 14.1 | DNA index 1.16 | Bone marrow and CNS | 100 | 80 | 21 | Alive, second CR, 12 mo |
CR, complete remission.
Kaplan-Meier analyses of outcomes in children with low-risk ALL receiving deintensified treatment. The 5-year rates are given as means ± standard error. CIR, cumulative incidence of relapse.
Kaplan-Meier analyses of outcomes in children with low-risk ALL receiving deintensified treatment. The 5-year rates are given as means ± standard error. CIR, cumulative incidence of relapse.
Discussion
In this study, we demonstrated that long-term survival rates exceeding 90% could be obtained in ∼25% of children with B-ALL treated with a mildly myelosuppressive chemotherapy regimen. All patients experienced complete remission, and there were no early deaths during remission induction. The overall rate of death resulting from toxicity was <1%. All patients completed therapy, and the median follow-up time was 6.6 years; therefore, the survival rates are unlikely to change substantially.8 The immunophenotypic and genotypic characteristics of B-ALL in this Brazilian cohort were similar to those seen in children with ALL in North American, European, and Asian countries.1,4-6,19 However, the frequency of different ALL subgroups in certain ethnic groups has not been fully characterized.20-22
The impetus for implementing a low-intensity regimen for patients with ALL in Recife came from the observation that a simplified flow cytometric assay revealed a subset of patients with B-ALL who had an exceedingly low cumulative risk of relapse when treated with standard chemotherapy regimens.13,23 The survival rates for our patients with VRL ALL were comparable to those for patients with low-risk B-ALL treated with much more intensive regimens.4-6,19,24 These observations suggest that by using a simple selection procedure, ∼25% of children with B-ALL can be treated with a relatively nontoxic regimen.
To reduce early mortality, all patients started remission induction with prednisone alone. The objective was to allow sufficient time to stabilize the patients and treat any active infectious and metabolic complications. Delaying the first intrathecal course was not associated with an increase in CNS relapse in these patients, as has also been observed in other studies.25,26 Notably, no patient had CNS 2 status, and only 1 of 10 patients with a traumatic tap had leukemia cells in the CSF. Using prednisone before the initial lumbar puncture might reduce the likelihood of CSF contamination with leukemia cells. These clinical observations are consistent with the recently proposed mechanism of leukemia penetration into the CNS.27
Eliminating the intensification from the remission induction did not adversely influence outcomes, given that the cumulative incidence of relapse in our study was similar to that in studies in which more intensive chemotherapy was administered during the early phases of treatment.4,6,19,24,28 The 4-hour infusion time for HD-MTX has been extensively used in Recife for patients with Burkitt lymphoma. We decided to maintain this schedule because of the lack of laboratory facilities to measure MTX levels and concerns about increased toxicity with prolonged MTX infusion. The MTX dosage and schedule used to treat ALL have been extensively investigated but remain controversial.29 The MRC trial5 eliminated HD-MTX in consolidation for patients with low-risk ALL but included 1 or 2 blocks of intensive chemotherapy containing anthracycline or cyclophosphamide. Despite our reducing the MTX intensity in consolidation without compensating for this with intensification blocks, the outcomes of our study patients were outstanding.
We cannot definitively state whether our criteria for selecting patients for reduced-intensity therapy can be further refined or whether ALL with MRD levels between 0.01% and <0.1% early in remission induction can be eradicated with low-intensity regimens. However, ALL with MRD levels between 0.001% and <0.01% at the end of induction (day 46) have been associated with an increased risk of relapse in St. Jude studies that used standard-intensity chemotherapy.2,30
One of our concerns was whether the efficacy of our regimen would be compromised by the treatment interruptions or modifications that often occur during treatment of ALL, such as those resulting from fever, neutropenia, allergic reactions, or other treatment-limiting toxicities. Admissions because of episodes of fever and neutropenia were common in all treatment phases, underpinning the importance of educating parents to follow a specific procedure to detect and respond to fever episodes and of ensuring the uninterrupted availability of medical services for the patients. The duration of neutropenia was usually short and uncomplicated, except in 1 patient, who died as a result of infectious complications. There was no association between relapse and treatment interruptions because of fever, neutropenia, or modifications to the L-asparaginase or daunorubicin dose intensity.
Although weekly oral MTX at home and monthly medical visits during the continuation phase have been standard practice,5 we opted to deliver MTX parenterally weekly at our outpatient clinic. The weekly contact with the family allowed us to detect and intervene in cases at high risk of infection or abandonment. Moreover, the weekly visits offered opportunities to educate parents at all treatment phases. This approach led to treatment-related mortality rates similar to those in published trials5,6 and the elimination of treatment abandonment.
The local medical team prepared a clinical summary for each new patient and presented the findings in a group discussion with St. Jude physicians weekly via online meetings. Grade ≥3 nonhematologic toxicity and considerations to hold chemotherapy were discussed immediately via e-mail or telephone contact. The local physicians were ultimately responsible for deciding whether to implement the recommendations.
A direct comparison of our protocol results with those reported in other low- to middle-income regions31,32 is not feasible because of the differences in the risk stratification criteria, treatment intensity, and frequency of competitive causes of treatment failure (abandonment and death resulting from toxicity). In our study, we had no cases of abandonment, and the rate of treatment-related deaths was low, reflecting the strategy used by the Recife medical team,10 which is focused on education and training for health care professionals, delivery of care in a multidisciplinary model, socioeconomic support for families, and close monitoring of patients during treatment to enable early detection of complications and prompt intervention.
Although the number of patients with highly chemotherapy-sensitive leukemia is relatively small (accounting for ∼27% of B-ALLs), the children classified as having intermediate-risk B-ALL and day-19 MRD between 0.01% and <0.1% had survival rates close to 90% with the modestly increased intensity of our low–dose intensity protocol (data not shown). For the children with intermediate-risk ALL and day-19 MRD of ≥0.1% or high-risk features, increasing the intensity of conventional chemotherapy regimens to obtain survival levels comparable to those obtained for patients with VLR or intermediate-risk ALL and MRD of <0.1% might be associated with prolonged myelosuppression and excessive treatment-related deaths. Integrating new classes of drugs, such as BCL-2 inhibitors33 and immunoconjugates,34 into low-intensity chemotherapy regimens may decrease the rates of relapse and death resulting from toxicity in low- and middle-income countries.
In summary, this study provides a proof of concept that a regimen with relatively low myelosuppressive activity can eradicate a subset of B-ALLs. Patients with highly sensitive ALL can be selected based on the presenting clinical and biologic features and on the degree of early response as measured by a simplified flow cytometric assay.
For original data, please contact raul.ribeiro@stjude.org.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Vani Shanker, ELS, and Keith Laycock, Editor in the Life Sciences (ELS), for editing the manuscript, and Ianne Feitosa for assisting with data collection.
This study was partially funded by National Cancer Institute, National Institutes of Health, grant CA21765 and by the American Lebanese and Syrian Associated Charities (R.C.R., G.K.R.).
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Authorship
Contribution: F.P., G.K.R., R.C.R., E.C.-S., and D.C. conceptualized and developed the study; F.P., A.P., M.M.L., M.P., N.L.-S., A.M.d.L.R., and E.V. provided study materials or patients; F.P., A.P., M.M.L., M.P., N.L.-S., A.M.d.L.R., and E.V. collected and assembled data; F.P., G.K.R., R.C.R., E.C.-S., D.C., Y.Z., and C.C. analyzed and interpreted data; F.P., G.K.R., and R.C.R. wrote the manuscript; and all authors revised and gave final approval for the manuscript, and were accountable for all aspects of the work.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Raul C. Ribeiro, Departments of Oncology and Global Pediatric Medicine, St. Jude Children’s Research Hospital, 262 Danny Thomas Place, Memphis, TN 38105; e-mail: raul.ribeiro@stjude.org.
REFERENCES
Author notes
F.P., G.K.R., D.C., and R.C.R. contributed equally to this work.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal