In this issue of Blood, Six et al provide evidence for the existence of myeloid- and lymphoid-dominant human hematopoietic stem and progenitor cells (HSPCs) using clonal tracking in patients treated with gene therapy for Wiskott-Aldrich syndrome (WAS) and β-hemoglobinopathies.1
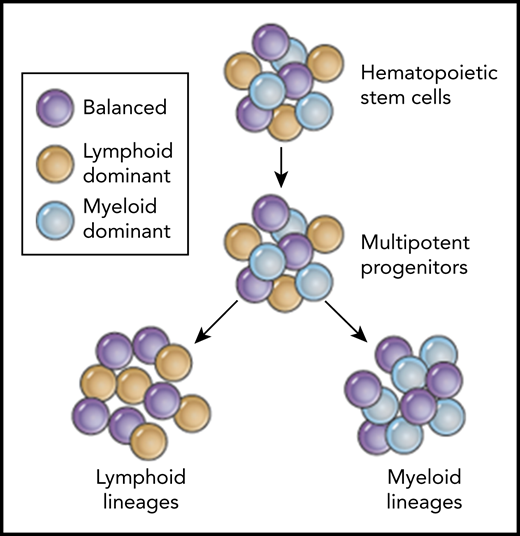
Predicted coexistence of balanced (purple), lymphoid-dominant (yellow), and myeloid-dominant (blue) human HSPCs based on clonal tracking data from patients after HSPC gene therapy.
Predicted coexistence of balanced (purple), lymphoid-dominant (yellow), and myeloid-dominant (blue) human HSPCs based on clonal tracking data from patients after HSPC gene therapy.
The analysis of retroviral integration sites after HSPC gene therapy has been originally developed to assess the safety of viral vectors for clinical applications. Integration site analysis (ISA) allows for the precise localization of insertions in the genome and provides a tool for the longitudinal assessment of clonality in engrafting cell populations after gene modification and transplantation. Triggered by the insertional mutagenesis-associated adverse events in gene therapy trials in the 1990s,2 this technology has become a central benchmark for integrating vector systems to determine the safety and to track integration sites in patients.
Beyond these important safety aspects, researchers quickly discovered the value of ISA to study hematopoietic reconstitution after transplantation, determine engraftment of true multilineage hematopoietic stem cells (HSCs), and dissect the lineage relationships among mature blood types. The steady decline in costs for next-generation sequencing, the development of cutting-edge bioinformatics tools, and especially, the availability of primary patient material from gene therapy trials have led to a remarkable series of discoveries in basic human HSPC biology over the past decade. A number of laboratories, including those of Bushman,2 Biasco,3 von Kalle and Schmidt,4 and Kiem,5,6 among others, have been finetuning the methods for ISA and optimizing the bioinformatic pipelines to handle these valuable patient samples and data. However, scarce patient material remains a bottleneck, limiting the number of repeat sequencing runs in order to reduce the variability, increase data quality, and, consequently, guarantee the correct interpretation of such complex datasets.
In this study, Six et al analyzed the long-term contribution of vector-modified HSPCs in 4 patients with WAS and 2 patients with β-hemoglobinopathies after successful gene therapy with lentiviral vectors. The authors tackled a variety of technical difficulties that can impact the quality of integration site sequencing data and easily lead to false interpretation of the clonal composition. The group sort-purified all 5 blood lineages from the peripheral blood of these patients, accounted for the cross-contamination during sampling and analysis, as well as incorporated the variance of the vector copy number within each lineage. This enormous effort not only allowed the authors to significantly reduce the known variability within ISA data but also at the same time enabled them to more stringently dissect the data and tease apart the contribution of individual clones to all 5 blood lineages.
With this more stringent and improved bioinformatics pipeline, the group made some interesting and important discoveries in their dataset. Similar to recent reports in mice7 and nonhuman primates (NHPs),8 Six et al identify individual clones that preferentially contribute to either lymphoid or myeloid lineages. The existence of such lymphoid-biased progenitors with long-term engraftment potential has recently been reported by Biasco et al in a clinical trial of HSPC gene therapy for WAS.9 However, in contrast to Biasco’s interpretation, Six et al suggest that such progenitors can be present for both lymphoid-dominant HSPCs and myeloid-dominant HSPCs, and that these lineage-biased progenitors can coexist within a bigger pool of unbiased multipotent HSPCs (see figure). The question of whether these lineage-dominant clones are the result of the actual transplant process or whether they also exist in an unperturbed human hematopoiesis as shown by Sun et al7 in mice has been the topic of active discussions in the hematopoiesis field. Recent studies in the mouse by the Weissman laboratory suggest that some of these differences can indeed be the result of different transplantation conditions and conditioning regimens.10 Thus, there remain plenty of unanswered questions in this rapidly evolving research area, but the findings by Six et al add to the growing body of studies challenging the originally proposed hierarchical lineage-relationships in human hematopoiesis and add valuable patient data to ongoing discussions.
Insights from this publication into human HSC biology aid to eventually close a long-standing gap between human hematopoiesis and the basic/preclinical HSPC research in animal models, such as the mouse or NHP, where lineage-dominant HSPCs have already been described. Findings in this publication significantly strengthen the translational potential of currently developed strategies in such animal models for the treatment of various hematological diseases and disorders. Although we are still not able to reliably identify such lymphoid- or myeloid-dominant human HSPC subsets using a defined set of cell surface markers as it is possible in the murine system, the potential targeting of these subsets for the treatment of specific diseases (such as immunodeficiencies or hemoglobinopathies) could be of clinical relevance.
Thus, with their improved processing and bioinformatics pipeline for vector-mediated ISA, Six et al have raised the bar and created a new standard for the analysis of such complex clonal tracking datasets in patients after HSPC gene therapy. Using their improved analysis technology, we can expect to further deepen our insights into human HSPC biology and hematopoiesis after transplantation.
Conflict-of-interest disclosure: H.-P.K. is a consultant for Rocket Pharma, Homology Medicines, CSL Behring, and Magenta Therapeutics. S.R. declares no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal