In this issue of Blood, Mollé et al demonstrate that on-target effects of ruxolitinib, a JAK1/2 inhibitor, reduce JAK2-mediated phosphorylation of STAT3 in the arcuate nucleus of the murine hypothalamus in response to feeding or exogenous leptin, proposing a novel mechanism for the weight gain observed with this drug.1
Leptin production from adipose tissue occurring as a satiety feedback signal is blocked by ruxolitinib in the arcuate nucleus of the hypothalamus. This interruption to a key component of appetite regulation may explain the weight gain commonly observed in ruxolitinib-treated patients.
Leptin production from adipose tissue occurring as a satiety feedback signal is blocked by ruxolitinib in the arcuate nucleus of the hypothalamus. This interruption to a key component of appetite regulation may explain the weight gain commonly observed in ruxolitinib-treated patients.
Ruxolitinib has become an established treatment in the myeloproliferative neoplasm (MPN) clinic. Inhibition of the JAK/STAT pathway in MPNs, where constitutive activation of this pathway is a key aspect of pathophysiology, has not yielded the same dramatic results as inhibition of BCR-ABL signaling in chronic myeloid leukemia. However, there are still many clear benefits for patients. Along with the objective benefits observed in myelofibrosis and polycythemia, including reduction in splenomegaly and improved hematocrit control, the reduction of a high symptom burden and improved quality of life are often reported by patients on therapy.2,3 The treatment, however, is not without adverse effects, and weight gain is frequently observed.4 In the cachectic patient with advanced myelofibrosis, ruxolitinib-induced weight gain is often considered beneficial.
Mollé et al identified 179 MPN patients in their institution using ruxolitinib for a median of 120 weeks and saw weight gain in more than two-thirds of patients on the drug. Based on their observation of weight gain across all MPN disease phenotypes, in patients with pretreatment low, normal, and high body mass index, it is clear that education about the risk of weight gain is relevant to all patients starting ruxolitinib and, likely by extension, any JAK2 inhibitor in the MPN clinic. Early weight change of >2% in the first 60 days was shown to predict for ongoing persistent weight gain in this cohort. This may help to predict the at-risk group of patients in whom more targeted interventions may be required to reduce the risk of further inappropriate weight gain and avoid any paradoxical increase in cardiovascular risk profile with treatment.
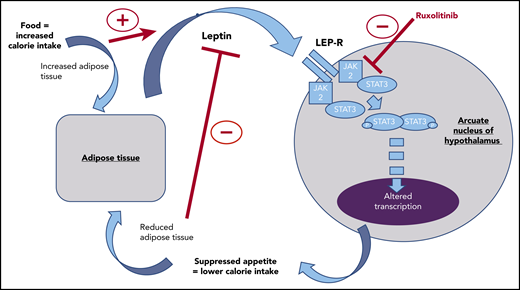
The regulation of appetite is complex. It involves interaction between neuronal circuits driving food seeking and rewarding food consumption, hormonal feedback mechanisms, and interactions with the gut microbiome.5-7 Leptin is a hormone produced by adipose tissue in response to sufficient calorie intake, acting through the leptin receptor in the arcuate nucleus of the hypothalamus as a signal of satiety (see figure). Understanding that JAK/STAT signaling is a key downstream pathway of the leptin receptor,5 Mollé et al used a murine model to demonstrate that the administration of ruxolitinib reduces the normal phosphorylation of STAT3 in the neuronal cells of the arcuate nucleus in the mouse hypothalamus seen in response to feeding or administration of exogenous leptin. In doing so, they demonstrated that ruxolitinib can interrupt the normal hormonal feedback mechanisms regulating appetite in the mouse. This, they suggest, could contribute to increased calorie intake and thus the weight gain seen in our patients.
JAK/STAT signaling pathways are critical in hematopoiesis, immune cell function, and many metabolic pathways. Therefore, unraveling the effects of JAK inhibition and attributing an effect to one process is complicated. In myelofibrosis, there is clear dysregulated enhanced production of proinflammatory cytokines driving catabolic processes.8 Ruxolitinib can mitigate the production and signaling of many of these cytokines,9 and this may also play an important role in the weight gain experienced by cachectic myelofibrosis patients. It is interesting to note, however, that in this real-world cohort, only 4% of patients were classified as underweight at time of drug initiation. This suggests that a majority of patients treated with ruxolitinib do not fit the cachectic phenotype. Across wider MPN phenotypes, which are infrequently associated with cachexia, the mechanism proposed by Mollé et al could have a significant role in weight gain.
Of course, cachexia can commonly result from numerous causes, malignant or otherwise, and is often a feature of frailty. Therefore, this work highlights both the need for vigilance regarding weight gain in the MPN clinic and the potential therapeutic benefit of manipulating leptin feedback via JAK/STAT signaling to stimulate appetite in the underweight/cachexic patient. More work will be required to explore this thought.
Conflict-of-interest disclosure: M.F.M. declares advisory board and speakers’ bureau participation for Novartis and Celgene. G.G. declares no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal