Acute myeloid leukemia (AML) cells highly depend on oxidative phosphorylation (OxPhos) to satisfy their heightened demands for energy, and the complex I OxPhos inhibitor IACS-010759 (Molina, Nat. Med. 2018) is currently in Phase 1 clinical trial in AML. In this study, we investigated how the bone marrow (BM) microenvironment affects the response to OxPhos inhibition in AML.
To characterize the molecular mechanisms of sensitivity to OxPhos inhibition, we performed Cap Analysis of Gene Expression analysis (CAGE) on 31 genetically diverse primary AML samples (20 were defined as sensitive and 11 as resistant to IACS-010759; cut off >3.0 fold annexin V(+) by 100 nM IACS-010759/DMSO at 72 hours). CAGE identified higher expression of transcription start sites (TSS) for 17 genes in IACS-010759 resistant AML samples compared to sensitive (fold change >2.0, FDR < 0.05, EdgeR), which were related to cell adhesion, integrin and/or Rho GTPase family genes that modulate intracellular actin dynamics.
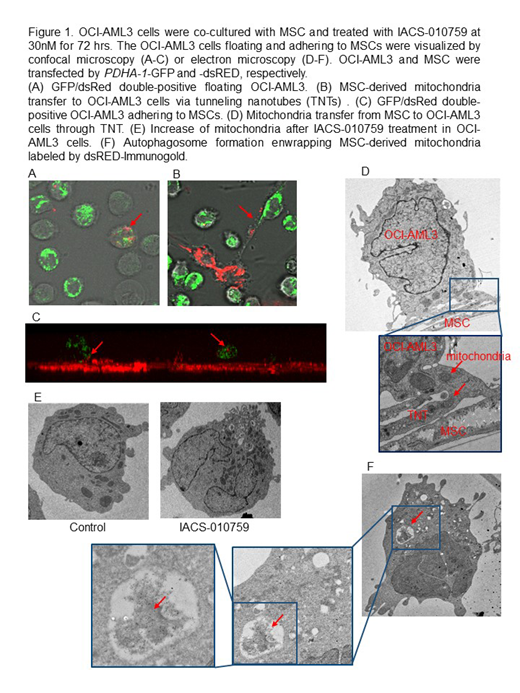
We next investigated the interactions between IACS-010759 sensitive OCI-AML3 cells and BM-derived mesenchymal stem cells (MSC). Under conditions mimicking the BM microenvironment, IACS-010759 upregulated the pathways of focal adhesion and ECM-receptor interaction in OCI-AML3 cells (KEGG analysis based on CAGE). In turn, MSC co-culture increased oxygen consumption by AML, induced generation of mitochondrial ROS (control 4.4% vs IACS 44.4%), increased mtDNA (2-fold by q-PCR) and upregulation of mitochondrial proteins VDAC and cytochrome C, translating into dampened growth-inhibitory effects of IACS-010759. We further demonstrated that OCI-AML3 cells adhering to MSCs were fully protected from IACS-010759 induced apoptosis (IACS-induced specific apoptosis: non-adherent cells 16.2% ± 1.6% vs adherent cells 1.6% ± 0.7%, p=0.008, 30nM, 72hours). Similarly, adherent cells were fully protected from apoptosis induced by combination of IACS and AraC. These findings indicate that direct interactions with MSC trigger compensatory activation of mitochondrial respiration, increase in mitochondrial mass and resistance to OxPhos inhibition in AML.
We next hypothesized that the trafficking of mitochondria from BM stroma cells to AML cells could represent a putative mechanism of an acquired resistance to OxPhos inhibition. To visualize mitochondria, OCI-AML3 and MSC were stably transfected with mitochondria-targeted PDHA1-GFP and -dsRed, respectively. We discovered that IACS-010759 induced transfer of MSC-derived mitochondria to OCI-AML3 cells (% of GFP/dsRed double-positive OCI-AML, control 4.1 ± 1.7 vs IACS 26.2 ± 13.4, p=0.002) via tunneling nanotubes (TNTs) detected by confocal and electron microscopy (Fig.1). Mitochondria transfer was only observed in the direct contact but not in the transwell co-cultures, and was abrogated by ICAM-1 neutralizing antibody and TNT blockade with Cytochalasin B. Likewise, combination of IACS with AraC increased mitochondrial transfer. We further found that IACS-010759 induced autophagy in OCI-AML3 cells co-cultured with MSC, as noted by increased conversion of LC3-I to LC3-II, which was further enhanced by the lysosome inhibitor Bafilomycin. Additionally, we observed autophagosome formation enwrapping MSC-derived mitochondria (Fig.1F), along with the degradation of an outer mitochondrial membrane protein Tom20.
Finally, IACS-010759-induced transfer of mtDNA in BM-resident AML cells was confirmed in vivo in humanized AML PDX models (n=2). Daily oral treatment of mice harboring human AML with IACS-010759 (5.0 mg/kg/day, 21 days) increased the ratio of murine/human mtDNA in human AML cells isolated from BM, in 5 days on/2 days off PDX models tested (2.1 ± 0.3 fold, n=2).
In conclusion, the findings of this study indicate an important role of mitochondria trafficking from BM stromal cells to AML cells in a compensatory adaptation to OxPhos inhibition in BM microenvironment. We propose that blocking of mitochondrial transfer could enhance the anti-AML efficacy of OxPhos targeting agents.
Zhang:The University of Texas M.D.Anderson Cancer Center: Employment. Kuruvilla:The University of Texas M.D.Anderson Cancer Center: Employment. Andreeff:BiolineRx: Membership on an entity's Board of Directors or advisory committees; Breast Cancer Research Foundation: Research Funding; Oncolyze: Equity Ownership; Oncoceutics: Equity Ownership; Senti Bio: Equity Ownership, Membership on an entity's Board of Directors or advisory committees; Eutropics: Equity Ownership; Reata: Equity Ownership; Aptose: Equity Ownership; 6 Dimensions Capital: Consultancy; Daiichi Sankyo, Inc.: Consultancy, Patents & Royalties: Patents licensed, royalty bearing, Research Funding; Jazz Pharmaceuticals: Consultancy; Celgene: Consultancy; Amgen: Consultancy; AstaZeneca: Consultancy; CPRIT: Research Funding; NIH/NCI: Research Funding; Center for Drug Research & Development: Membership on an entity's Board of Directors or advisory committees; Cancer UK: Membership on an entity's Board of Directors or advisory committees; NCI-CTEP: Membership on an entity's Board of Directors or advisory committees; German Research Council: Membership on an entity's Board of Directors or advisory committees; Leukemia Lymphoma Society: Membership on an entity's Board of Directors or advisory committees; NCI-RDCRN (Rare Disease Cliln Network): Membership on an entity's Board of Directors or advisory committees; CLL Foundation: Membership on an entity's Board of Directors or advisory committees. Konopleva:Astra Zeneca: Research Funding; Agios: Research Funding; Eli Lilly: Research Funding; AbbVie: Consultancy, Honoraria, Research Funding; Cellectis: Research Funding; Amgen: Consultancy, Honoraria; F. Hoffman La-Roche: Consultancy, Honoraria, Research Funding; Genentech: Honoraria, Research Funding; Ascentage: Research Funding; Kisoji: Consultancy, Honoraria; Reata Pharmaceuticals: Equity Ownership, Patents & Royalties; Ablynx: Research Funding; Forty-Seven: Consultancy, Honoraria; Calithera: Research Funding; Stemline Therapeutics: Consultancy, Honoraria, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal