Introduction: Daratumumab (DARA) is a human IgGκ monoclonal antibody targeting CD38 with a direct on-tumor and immunomodulatory mechanism of action. The addition of DARA to standard-of-care regimens in phase 3 studies reduced the risk of disease progression or death by ≥44%, nearly doubled the rate of complete response or better, and induced a ≥3-fold increase in minimal residual disease (MRD)-negativity rates versus standard of care alone in patients with transplant-ineligible newly diagnosed multiple myeloma (NDMM) and relapsed/refractory multiple myeloma. In the primary analysis of the phase 3 ALCYONE study (median follow-up: 16.5 months), a significant progression-free survival (PFS) benefit (median not reached vs 18.1 months; hazard ratio [HR], 0.50; P <0.001) was observed with the addition of DARA to bortezomib/melphalan/prednisone (D-VMP) in patients with transplant-ineligible NDMM, without an increase in overall toxicity (Mateos MV, et al. N Engl J Med. 2018;378[6]:518-528). D-VMP continued to demonstrate a significant PFS benefit versus VMP alone after 1 year of additional follow-up, including in patients ≥75 years of age (Dimopoulos MA, et al. Blood. 2018;132[Suppl 1]:156). After a median follow-up of 27.8 months, D-VMP reduced the risk of disease progression or death by 57% versus VMP alone, with a 24-month PFS rate of 63% in the D-VMP group and 36% in the VMP group. This PFS benefit was observed regardless of patient age and was maintained during the subsequent line of therapy in patients with transplant-ineligible NDMM. Here, we present >36 months of follow-up from ALCYONE, including analysis of overall survival (OS) from a prespecified interim analysis.
Methods: Patients with NDMM ineligible for high-dose chemotherapy and autologous stem cell transplantation due to age (≥65 years) or comorbidities were randomized 1:1 to receive up to nine 6-week cycles of VMP (bortezomib 1.3 mg/m2 subcutaneously on Days 1, 4, 8, 11, 22, 25, 29, and 32 of Cycle 1 and Days 1, 8, 22, and 29 of Cycles 2-9; melphalan 9 mg/m2 orally and prednisone 60 mg/m2 orally on Days 1-4 of Cycles 1-9) with or without DARA (16 mg/kg intravenously once weekly for Cycle 1, once every 3 weeks for Cycles 2-9, and once every 4 weeks for Cycles 10+ until disease progression). The primary endpoint was PFS. Secondary endpoints included overall response rate, rate of complete response or better, rate of very good partial response or better, MRD-negativity rate (10-5 threshold), PFS on subsequent line of therapy (PFS2), OS, and safety.
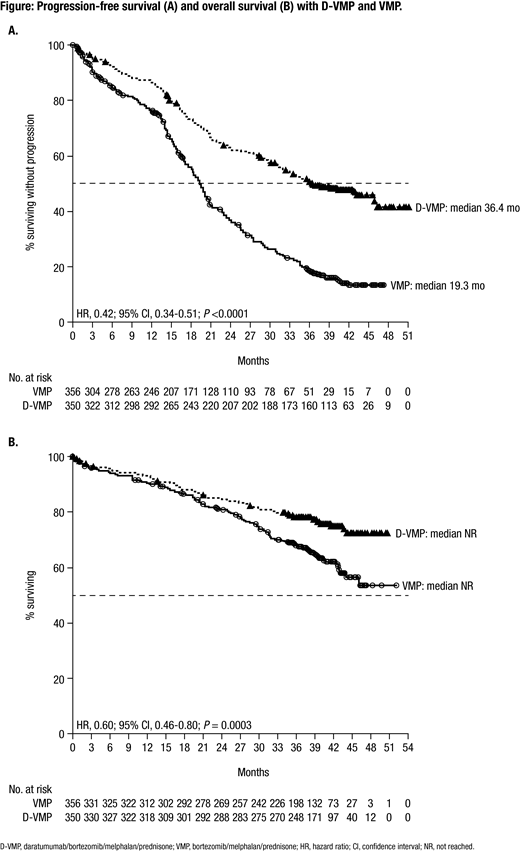
Results: A total of 706 patients were enrolled in this study (D-VMP: n = 350; VMP: n = 356). Patient baseline characteristics were well balanced between treatment arms. The median (range) age was 71 (40-93) years, and 29.9% of patients were ≥75 years of age. 518 (84.1%) and 98 (15.9%) of 616 patients evaluated had standard and high (del17p, t[14;16], and/or t[4;14] positive) cytogenetic risk, respectively, as assessed via local fluorescence in-situ hybridization/karyotyping. Median PFS was 36.4 months with D-VMP versus 19.3 months with VMP after a median follow-up of 40.08 months (HR, 0.42; 95% confidence interval [CI], 0.34-0.51; P <0.0001; Figure A). Median PFS2 was not reached with D-VMP versus 42.3 months with VMP (HR, 0.55; 95% CI, 0.43-0.71; P <0.0001). The estimated 36-month OS rate was 78% with D-VMP versus 68% with VMP, with a significant benefit for OS observed for D-VMP versus VMP alone (HR, 0.60; 95% CI, 0.46-0.80; P = 0.0003; Figure B); median OS was not reached in either group and follow-up is ongoing. Additional efficacy data, including MRD negativity, and updated safety data will be presented at the meeting.
Conclusions: For the first time, we demonstrate that the addition of DARA to VMP prolongs OS in patients with transplant-ineligible NDMM, with a 40% reduction in the risk of death versus VMP alone after a median follow-up of 40 months. D-VMP continued to demonstrate a significant PFS benefit, which was also maintained during the subsequent line of therapy. These findings, together with the phase 3 MAIA study (DARA plus lenalidomide/dexamethasone vs lenalidomide/dexamethasone), continue to support the addition of DARA to frontline treatment regimens in patients with transplant-ineligible NDMM.
Mateos:Abbvie: Membership on an entity's Board of Directors or advisory committees; GSK: Membership on an entity's Board of Directors or advisory committees; EDO: Membership on an entity's Board of Directors or advisory committees; Pharmamar: Membership on an entity's Board of Directors or advisory committees; Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees; Takeda: Honoraria, Membership on an entity's Board of Directors or advisory committees; Amgen: Honoraria, Membership on an entity's Board of Directors or advisory committees; Celgene: Honoraria, Membership on an entity's Board of Directors or advisory committees; Adaptive: Honoraria. Cavo:Janssen, Celgene, Amgen, Abbvie: Consultancy, Membership on an entity's Board of Directors or advisory committees; Celgene, Janssen, Amgen, BMS, Abbvie, Takeda: Honoraria; Janssen, Celgene: Other: Travel Accommodations; Janssen, Celgene: Speakers Bureau. Bladé:Irctures: Honoraria; Janssen, Celgene, Amgen, Takeda: Membership on an entity's Board of Directors or advisory committees. Dimopoulos:Sanofi Oncology: Research Funding. Suzuki:Ono: Research Funding; BMS: Honoraria, Research Funding; Takeda: Honoraria; Janssen: Honoraria; Celgene: Honoraria. Jakubowiak:Janssen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; KaryoPharm Therapeutics: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Takeda: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Juno: Consultancy, Honoraria; Adaptive Biotechnologies: Consultancy, Honoraria; Celgene: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Sanofi: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; SkyLineDx: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; BMS: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; GSK: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Amgen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; AbbVie: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Millennium: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees. Knop:Janssen, AMGEN, Bristol-Myers Squibb, Celgene: Consultancy, Honoraria. Lucio:Janssen: Consultancy, Honoraria; Celgene: Consultancy, Honoraria; Takeda: Consultancy, Honoraria; AbbVie: Consultancy, Honoraria. Nagy:Abbvie: Membership on an entity's Board of Directors or advisory committees; Roche: Membership on an entity's Board of Directors or advisory committees; Janssen: Membership on an entity's Board of Directors or advisory committees; Novartis: Membership on an entity's Board of Directors or advisory committees; Takeda: Membership on an entity's Board of Directors or advisory committees. Cook:Celgene, Jannsen-Cilag, Takeda: Honoraria, Research Funding; Amgen, Bristol-Myers Squibb, GlycoMimetics, Seattle Genetics and Sanofi: Honoraria. Liberati:Janssen: Honoraria; Bristol & Mayer: Honoraria; Celgene: Honoraria; Servier: Honoraria, Membership on an entity's Board of Directors or advisory committees; Takeda: Membership on an entity's Board of Directors or advisory committees; Amgen: Membership on an entity's Board of Directors or advisory committees; AbbVie: Honoraria, Membership on an entity's Board of Directors or advisory committees; Incyte: Consultancy. Campbell:Janssen: Honoraria, Research Funding, Speakers Bureau. Garg:Novartis, Janssen: Research Funding; Janssen, Takeda, Novartis: Other: Travel expenses; Janssen: Honoraria. Krevvata:Janssen: Employment. Wang:Janssen: Employment. Kudva:Janssen: Employment, Equity Ownership. Ukropec:Janssen: Employment, Equity Ownership. Wroblewski:Janssen: Employment, Equity Ownership. Kobos:Janssen: Employment. San-Miguel:Amgen, Bristol-Myers Squibb, Celgene, Janssen, MSD, Novartis, Roche, Sanofi, and Takeda: Consultancy, Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal