Introduction
CD19-targeting chimeric antigen receptor (CAR) T cell therapy has demonstrated high success; however, its therapeutic potential can still be further improved. In addition, the high cost and lengthy process of CAR-T production limit its broad application. We have developed a new platform termed FasT (F) CAR-T with shortened manufacturing time to one day (plus 7 days of additional testing for regulatory requirements). Here we report results from a pre-clinical study of FasT (F) CAR-T (GC007F) and a phase Ⅰ clinical trial to assess the safety and feasibility of treating patients with CD19+ relapsed/refractory B-cell acute lymphoblastic leukemia (B-ALL).
Methods
In this study, a second generation of CD19-directed CAR-T was manufactured using the FasT CAR-T platform. Peripheral blood (PB) mononuclear cells were obtained by leukapheresis either from healthy donors for the pre-clinical study or from patients undergoing the clinical trial. T cells were separated and used for CAR-T generation. A xenograft mouse model was used to determine the efficacy of GC007F in vivo. Conventional (C) CAR-T derived from the same healthy donor were also made and tested in parallel for comparison.
Between Feb. 2019 and July 2019, 10 adolescent and adult patients with CD19+ relapsed/refractory B-ALL were enrolled in a feasibility trial for CD19 FasT CAR-T (www.clinicaltrials.gov, NCT03825718). FasT CAR-T cells for all patients were successfully manufactured. All patients received a conditioning regimen of IV fludarabine (30mg/m2/d) and cyclophosphamide (250mg/m2/d) for 3 days followed by a single infusion of CAR-T cells. Six patients received a low-dose 6.5 (5.86-7.04) x104/kg of FasT CAR-T, 2 received a medium-dose 1 (1-1.16) x105/kg, and 1, a high-dose 1.56x105/kg. The primary end points of the study were to evaluate feasibility and toxicity, and the secondary end points included disease response and engraftment/persistence of infused FasT CAR-T cells.
Results
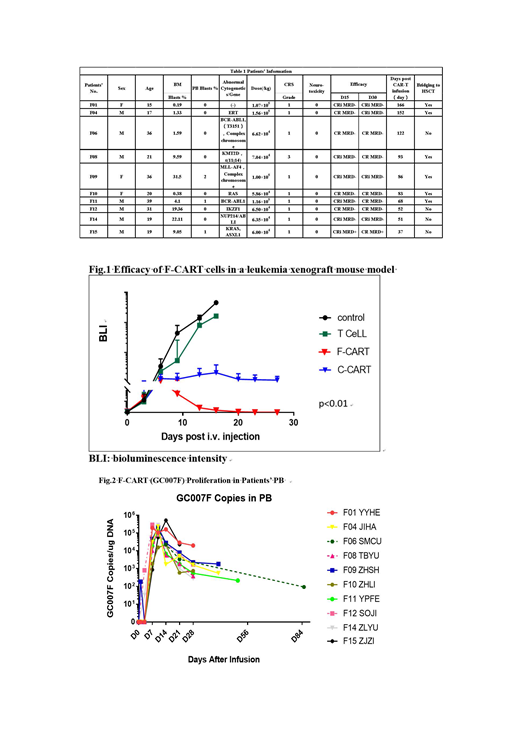
This preclinical study has demonstrated several significant improvements of CD19-directed F CAR-T over C CAR-T: 1) 5-30 fold superior expansion capability (p<0.01); 2) more abundant T central memory cells (Tcm) (73.47±2.85% vs 58.03±8.34%, p<0.05) and T memory stem cells (Tscm) (6.42±3.64% vs 0.39±0.13%, p<0.01); 3) less exhaustion with reduced levels of PD-1+ and LAG3+ (3.39±0.49% vs 12.66±1.87%, p<0.01); and 4) more effective in the elimination of B-ALL in a xenograft mouse model (p<0.01, Fig. 1).
For the phase Ⅰ clinical trial, the median observation period was 86 days (37-166 days). The median percentage of pre-treatment bone marrow (BM) blasts was 9.05% (0.19-32.5%). On day 15 after CAR-T cell infusion, 10/10 (100%) cases achieved complete remission (CR) or CR with incomplete count recovery (CRi) and 9/10 (90%) had minimal residual disease (MRD)-negative CR. Four of ten patients had a good blood count recovery on day 15. The number further increased to 6/10 on day 30. Patient F15 had rapidly growing disease in that his PB blasts increased from 1% on enrollment to 7% immediately before CAR-T cells infusion, and increased to 77% on day 7 post infusion. Notwithstanding the rapid disease progression, the patient achieved MRD-positive CR on day 15 with residual 0.06% BM blasts. Five of ten patients were bridged into allogeneic hematopoietic stem cell transplantation (allo-HSCT). All 10 patients have remained in CR thus far. After CAR-T infusion, the level of infused CD19 FasT CAR-T cells in PB was analyzed by qPCR and flow cytometry. Superior in vivo proliferation and persistence were detected regardless of the infused CAR-T doses. The median peak level was reached on day 7 (7-10) with 2.1(0.22-5.2) x105 copy/µg PB genomic DNA (Fig. 2) and the median CAR-T expression ratio was 44.5 (13.6-69.5) %. The peaks of IL6, IFNγ, IL10, and CD25 were observed around day 7. Despite the achievement of a very high CR rate, 9/10 had grade 1 cytokine release syndrome (CRS) and only 1 patient experienced grade 3 CRS. None developed neurotoxicity.
Conclusion
This study has demonstrated that FasT CAR-T cells with superior expansion capability and younger/less exhausted phenotypes can be generated rapidly. This first-in-human clinical study showed that FasT CAR-T is safe and highly effective for treating patients with B-ALL.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal