Background: HbF induction is a key therapeutic strategy for sickle cell disease (SCD). Analysis of whole exome sequencing (WES) data from patients with SCD identified variants in two components of the insulin signaling pathway, FOXO3 and its activator, AMPK, to be associated with HbF levels; the association was confirmed by functional studies in hematopoietic stem and progenitor cells (HSPC) (Zhang, Blood 2018). This work has led to a clinical trial of metformin, an activator of FOXO3, as a novel HbF inducing agent in patients with SCA (NCT02981329). We then performed whole genome sequencing (WGS) on 567 samples from patients with SCA, and identified an association between another component of the insulin signaling pathway, IGFBP3, and HbF levels (p<1x10-6). Of note, IGFBP-3 expression is upregulated by several drugs also reported to increase HbF, including decitabine, metformin, and vitamin D.
Methods: Plasma levels of IGFBP3 relative to IGF1 in patients with and without IGFBP3 variants were measured by ELISA. Three unique SCD patient-derived HSPC cultures were treated with metformin (100 µM), piceatannol (12.5 µM) compound C (1 µM), and exogenous IGFBP3 (1µg/ml); their effect on HbF, gamma-globin, known modifiers of HbF, protein levels and phosphorylation status of members of the FOXO3-AMPK pathway were assessed by HPLC, RT-qPCR and western blot at day 14 and 21 of culture.
Results
In vitro: Plasma IGFBP3 levels were higher in patients heterozygous for an IGFBP3 variant (p=0.01). Treatment of HSPCs with recombinant IGFBP3 resulted in a significant increase in %HbF (p=0.008). Adding IGFBP3 to erythroid culture altered the insulin signaling pathway; both total protein and activated phosphorylated (Ser 413) levels of FOXO3 increased (p=0.01 and p=0.03, respectively). Piceatannol induces HbF (Zhang, Blood 2018), however, this effect was abolished when AMPK specific inhibitor compound C was added (p=0.01). Neither IGFBP3 nor metformin altered erythroid maturation or expression of known gamma-globin regulators BCL11A, KLF1, and MYB; however, addition of IGFBP3 increased total NRF2 protein levels and Ser40 NRF2 phosphorylation.
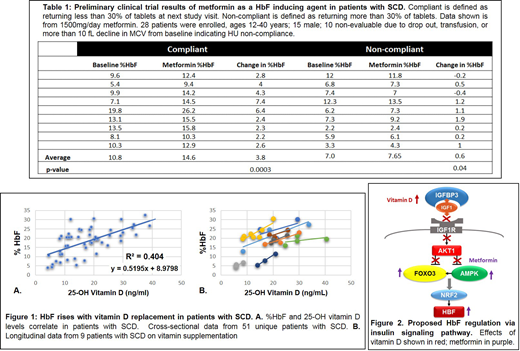
In vivo: In Table 1, we show the HbF response to metformin from our prospective clinical trial. Patients who demonstrated compliance with metformin showed an average 4 percentage point rise in HbF. Furthermore, retrospective chart review of HbF and vitamin D levels in patients with SCD indicate that HbF levels correlate strongly with vitamin D levels (R2=0.404), and that vitamin D supplementation increases HbF in patients with SCD (Figure 1).
Conclusions:
In vitro: We have shown that elevation or activation of IGFBP3, FOXO3, and AMPK induces HbF in HSPCs in vitro, without altering erythroid maturation or levels of BCL11A, KLF1, or MYB. These results show that manipulation of the insulin signaling pathway at several levels can induce HbF in vitro in HSPCs. We hypothesize that circulating IGFBP3 induces HbF via the insulin signaling pathway, by binding IGF1, preventing activation of the IGF1 receptor (IGF1R), a negative regulator of FOXO3. Thus, IGFBP3 may promote HbF production by inhibiting aFOXO3 inhibitor (Figure 2), and by activating a known positive regulator of HbF, NRF2.
In vivo: Preliminary results from our clinical trial of metformin in patients with SCA shows a rise in HbF in adherent patients, providing in vivo support for the role of the insulin signaling pathway in HbF regulation. Correlations between HbF and vitamin D levels in patients with SCD suggest that agents that increase IGFBP3 like vitamin D, may increase HbF in patients with SCD. Our in vitro and in vivo data in combination indicates a role for the insulin signaling pathway in HbF regulation. We propose that the insulin signaling pathway can be pharmacologically targeted with safe, well-studied agents like metformin and Vitamin D along with other HbF inducers to maximize clinical benefit.
Weiss:Cellarity INC: Consultancy; Rubius INC: Consultancy; GlaxoSmithKline: Consultancy; Esperian: Consultancy; Beam Therapeutics: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal