
OS for childhood ALL is now >90%, but high-risk (HR) cases still need to improve. The NOPHO ALL2008 protocol restricted very intensive HR chemotherapy to patients with an inferior early minimal residual disease (MRD) response or HR genetics, and we here report the results for this subset.
HR patients had WBC >100K at diagnosis and/or T-ALL; and an MRD of either >25% day 15 or >0.1% day 29; and/or a KMT2A-re or hypodiploidy (<45 chr). MRD was assessed by flow cytometry (BCP) or PCR (T-ALL). If no marker was found with one, the alternative method was used. Thus, >98% had a suitable MRD marker. All HR patients received a 3-drug induction chemotherapy (VCR 2 mg/m2 x5), DOX (40 mg/m2 x2), PRED (60 mg/m2/d for 4 weeks for BCP-ALL with WBC <100K) or DEXA (10 mg/m2/d for 3 weeks for all others). This was followed by 9 (A1-B1-C1-A2-B2-C2-A3-B3-C3) or 7 (if MRD after 1st HR block <0.1%; C2/C3 deleted) blocks, 60 weeks of interim maintenance with oral 6MP/MTX and HD-MTX x3 (5g/m2/d), delayed intensification, and maintenance therapy until a total of 2.5 years from diagnosis. Post-induction, a total of 12 doses of i.m. Peg-asp (1,000 IU/m2) was given (Toft, Leukemia 2018). A-blocks included cyclophosphamide (440 mg/m2/d x5), etoposide (100 mg/m2/d x5), and Peg-asp (1000 IU/m2). B-blocks consisted of HD-MTX (5g/m2), HD-AraC (2x2g/m2 /d x2), 6MP (100 mg/m2/d x5), DEXA (20 mg/m2/d x5), VCR (2 mg/m2 x2), Peg-asp (1000 IU/m2). C-blocks included idarubicin (8 mg/m2), fludarabine (30 mg/m2/d x5), HD-AraC (2g/m2 /d x5), Peg-asp (1000 IU/m2). Patients were allocated to hSCT if MRD >5% at end of induction, or >0.1% after 2 HR-blocks or ~3 months of LR-chemotherapy. HSCT-patients are not included here. Data on 18 toxic adverse events was prospectively collected at three-month intervals. The Kaplan-Meier method was used for OS and EFS analyses. CI of Rel (CIR) and TRM (CITRM) were estimated accounting for competing events. Cox-models were used to compute hazard ratios and proportional hazard assumptions were evaluated using Schoenfeld residuals.
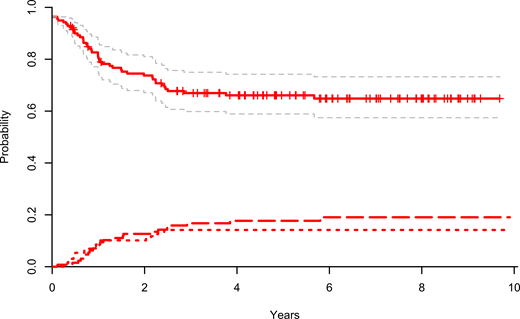
132 subjects 1-≤18 years of age at diagnosis were eligible for HR chemotherapy accounting for 16% of the study cohort with a median age of 6.6 years as compared to 4.6 years in the entire cohort. Of the HR chemotherapy subjects 26% received PRED in induction. Five-year EFS was 67% (95% CI, 59-76%) and OS was 74% (95% CI, 67-82%) in the HR chemo cohort. CIR at five years was 18% (95% CI, 11-24%). We found no difference in EFS by cell lineage (BCP-ALL: 69%, 95% CI, 59-80% vs. T-ALL 65%, 95% CI 52-77%). KMT2A-re occurred in 34 patients, 62% of whom had MRD <0.1% after induction. Their five-year EFS and OS were 88% (95% CI, 77-99%). KMT2A-re patients with a leukocyte count <100K and EOI MRD <0.1% had no relapses, but toxic deaths resulting in a 5-year EFS of 80% (95% CI, 55-100%). Hypodiploid patients had a five-year EFS and OS of 55% (95% CI, 34%-76%) and 58% (95% CI, 39-78%), respectively. Strikingly, KMT2A-re and hypodiploid patients with a negative MRD at EOI, suffered no relapses. 29 patients shifted to HR-blocks at day 15 due to MRD >25% (T-ALL (N=17), BCP with WBC >100K (N=7), KMT2A-re (N=4), or hypodiploid ALL (N=1). Their five-year EFS was 47% (95% CI, 21-73%) and CIR 44% (95% CI, 18-70%). During the HR chemotherapy blocks, 70% (N=93) of patients encountered at least one toxic event beyond bacteremia and febrile neutropenia, the most common of which was fungal infections (25%), referral to intensive care (23%), and asparaginase associated anaphylaxis (21%).
The NOPHO ALL2008 stratification strategy led to allocation of 16% of patients to the HR arm with an EFS of 67% and OS of 74%. Due to the intensity of chemotherapy a significant risk of toxic death was encountered, and the risks of toxic death and relapse were very similar. Patients with rearranged KMT2A and a low EOI MRD could be candidates for future reduction of treatment intensity. These findings are integrated into the upcoming European ALLTogether1 protocol opening in 14 European countries early 2020, where the use of the NOPHO ALL2008 HR blocks will be restricted to the 3% of patients with the poorest prognosis.
Figure - EFS with 95% Cis, CITRM (short dash) and CIR (long dash) for the NOPHO ALL-2008 high-risk chemotherapy patients
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal