Chemotherapies for cancers induce granulocytopenia and additively increase the risk of infection. Infections in patients with granulocytopenia, especially neutropenia, have remained as a major problem. Though granulocyte transfusion therapy (GTX) is a therapeutic option against neutropenic infections refractory to supportive therapies, GTX has not been in wide use mainly due to its physical burden on donors. We have previously reported the establishment of genetically engineered hematopoietic progenitors priming neutrophils (NeuP-HPCs) from human induced pluripotent stem cells (iPSCs) by inducible expression of c-MYC and BMI1, which cultured on OP9 feeder cells, kept expanding until 12 weeks after the induction and were differentiated into functional neutrophils four days after silencing c-MYC and BMI1. In the current study, we sophisticated the previous production system and established a robust and feeder-free production system of neutrophils, for the purpose of donor-free GTX.
To enhance the expandability of NeuP-HPCs to be preferably applied in clinical settings, we investigated genes enhancing the expandability of NeuP-HPCs. we performed single-cell analysis of focused 83 transcriptional factors in HPCs and identified the population committing neutrophils in early phase, which enriched the expression of BCL6, CEBPB, CEBPD, and LITAF. As CEBPB provided the two-weeks extension of the expandability among four genes and had a lot of target genes, we conducted the search for target genes of CEBPB which purely enhanced the expandability. Motif analysis of CEBPB revealed that BCL2A1 was the gene associated with the cell proliferation in top-ranked ten target genes, suggesting that BCL2 family genes enhanced the expandability of NeuP-HPCs. Consistent with the result, the expression of BCL2A1, BCL-XL or MCL1, which were the major BCL2 family genes in the development of myeloid cells, enhanced the expandability and kept expanding for at least 16 weeks, indicating that one NeuP-HPC was estimated to generate thousands aliquot of 5.0 x 1010 cells, clinically meaningful scale, in 10 weeks. Notably, NeuP-HPCs expressing BCL-XL (NeuP-HPCs-XL) achieved the feeder-free expansion, suggesting that NeuP-HPCs-XL were preferable to clinical application.
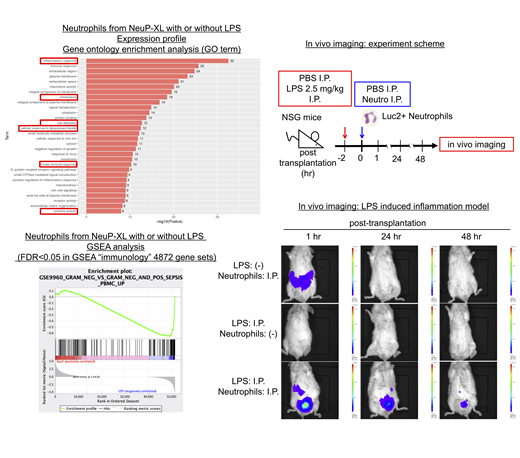
Whereas the constitutive expression of BCL2 family genes inhibited the neutrophilic differentiation, NeuP-HPCs-XL with the inducible expression of c-MYC, BMI1, and BCL-XL generated the functional neutrophils, which showed expression of neutrophil-specific markers, CD16b and CD66b, formed toxic granules with lipopolysaccharides (LPS) and produced reactive oxygen species (ROS) with phorbol-12-myristate-13 acetates (PMA). Gene expression profiling by RNA sequence comparing the neutrophils with or without LPS revealed the activation of NF-kB pathway, which was the critical pathway through LPS stimulation. Furthermore, the neutrophils with LPS stimulation recapitulated the expression profile of peripheral blood in patients with gram-negative sepsis. Gene ontology enrichment analysis showed the enrichment of inflammatory response, innate immune response, cytokine activity (including higher expression of IL1A, IL1B, IL6 and TNFa), cell adhesion (including higher expression of ICAM1, also a functional marker of phagocytosis) and chemotaxis (including higher expression of CXCL2, CXCL8, CCL19 and CCL20) with LPS stimulation. These findings indicated that the neutrophils from NeuP-HPCs-XL harbored the principal neutrophil functions including adhesion, chemotaxis, phagocytosis, ROS generation and cytokine production.
To assess the function of neutrophils from NeuP-HPCs-XL in vivo, we performed in vivo imaging of the neutrophils expressing luciferase in the mice model of LPS-induced inflammation. In NSG mice harboring the local inflammation induced by intraperitoneal injection of LPS, intraperitoneally administrated neutrophils aggregated specifically at the inflammation site and showed the persistent survival for 72 hours, in marked contrast to the diffuse distribution and the shorter survival for 24 hours in NSG mice without LPS injection. These findings suggested that neutrophils from NeuP-HPCs-XL were functional in vivo.
In summary, we achieved the feeder-free and robust production of neutrophils with the functional capacity in vivo. NeuP-HPCs-XL are potential sources of neutrophils for donor-free GTX.
Miyauchi:Kyowa Hakko Kirin Co. Ltd.: Research Funding. Iwasaki:Kyowa Hakko Kirin Co. Ltd.: Employment. Kawagoshi:Kyowa Hakko Kirin Co. Ltd.: Employment. Kagoya:Nihon Shinyaku: Research Funding; Kyowa Kirin: Speakers Bureau. Kurokawa:Nippon Shinyaku Co., Ltd.: Research Funding; Teijin Limited: Research Funding; Shire Japan K.K.: Speakers Bureau; Bristol-Myers Squibb: Speakers Bureau; Chugai Pharmaceutical Company: Consultancy, Research Funding, Speakers Bureau; ONO PHARMACEUTICAL CO., LTD.: Speakers Bureau; Bioverativ Japan ltd.: Consultancy; MSD K.K.: Consultancy, Research Funding, Speakers Bureau; Celgene K.K.: Consultancy, Speakers Bureau; Boehringer Ingelheim: Speakers Bureau; Kyowa Hakko Kirin Co., Ltd.: Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Daiichi Sankyo Conpany: Speakers Bureau; Sumitomo Dainippon Pharma Co.,Ltd.: Research Funding, Speakers Bureau; Janssen Pharmaceutical K.K.: Speakers Bureau; Shionogi & Co., Ltd: Consultancy, Honoraria; Otsuka Pharmaceutical Co., Ltd.: Research Funding, Speakers Bureau; Astellas Pharma Inc.: Research Funding, Speakers Bureau; Takeda Pharmaceutical Company Limited.: Research Funding, Speakers Bureau; Yakult Honsha Company: Speakers Bureau; Pfizer Japan Inc.: Research Funding; Eisai Co., Ltd.: Research Funding, Speakers Bureau; Novartis Pharma K.K.: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal