Introduction
Most blood antigen-carrying proteins have important functions not only in red blood cells (RBCs) but also in other tissues. Hence, because of their polymorphisms and the existence of natural null phenotypes, blood groups also represent powerful tools to investigate human diseases. The PEL-negative rare blood type is one of the last with an unknown genetic basis. Family studies showed that the PEL-negative phenotype was inherited as an autosomal recessive trait, but the PEL locus remained undetermined since the first description of the corresponding antibody in French-Canadian individuals in 1996.
Results
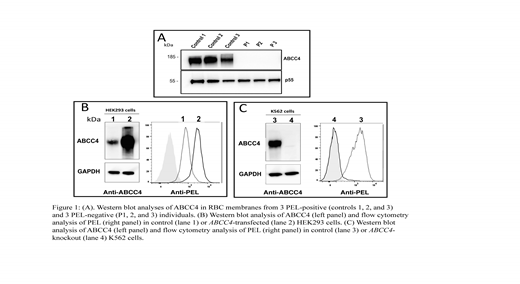
Combining whole-exome sequencing and comparative global proteomic approaches using genomic DNA and red cell membrane proteins from four unrelated French-Canadian PEL-negative individuals and from controls, we identified i) the Multidrug Resistance-Associated Protein 4 (MRP4), also known as ABCC4, as the only missing protein in all PEL-negative RBCs and ii) a large deletion removing the last 10 exons of the ABCC4 gene. Western blot analyses confirmed that ABCC4 was present in the membrane of PEL-positive control RBCs but totally absent in the PEL-negative RBCs (Fig 1A). Western blot and flow cytometry analyses of transfected HEK293 cells revealed that over-expression of ABCC4 results in a significant increase in PEL antigen cell-surface expression (Fig 1B). Conversely, similar analyses of ABCC4-knockout K562 cells generated by the CRISPR-Cas9 strategy showed that the complete loss of ABCC4 abolished the expression of the PEL antigen (Fig 1C).
PEL-negative individuals are therefore the first ever reported population lacking the complete expression of the ABCC4 protein. Surprisingly, despite the expected biological importance of ABCC4 as a cyclic nucleotide transporter, no apparent mutual symptoms or diseases were identified after investigation of the clinical history of the 12 PEL-negative members within the 4 families analyzed in this study. However, platelet investigation from one PEL-negative individual supported the role of this protein in the modulation of platelet aggregation, as previously observed in ABCC4-knockout mice studies
To better understand the function of ABCC4 in mature RBCs, we measured the intracellular cGMP level in RBCs from 5 PEL-negative individuals and 5 controls. We showed that the cGMP level was not significantly impaired in the absence of ABCC4 suggesting that another cGMP transporter could compensate for the absence of ABCC4 on the RBC membrane. Interestingly, proteomic and flow cytometry analyses indicated that among the five other ABC transporters (ABCC1, ABCA7, ABCB6, ABCC5 and ABCG2) expressed on RBCs, only ABCG2 was increased in PEL-negative/ABCC4null RBCs compared to controls (1.3-fold; p=0.0007). This suggested that the absence of ABCC4 in PEL-negative individuals could be compensated by the over-expression of ABCG2. Our data, together with previous studies indicating that both ABCC4 and ABCG2 export cGMP, strongly suggest that the over-expression of ABCG2 in PEL-negative RBCs represents a compensatory mechanism for the lack of ABCC4, which could explain the absence of hematological abnormalities in PEL-negative individuals.
Discussion
Using genetic, molecular and cellular approaches, we demonstrated that ABCC4 is the gene underlying the novel PEL blood group system and that homozygosity for a large deletion in this gene is responsible for the rare PEL-negative phenotype. ABCC4 represents the third ABC transporter carrying a blood group system after ABCG2 (JR) and ABCB6 (LAN). The expression level of ABCC4 is strongly involved in drug resistance and toxicity, and in the clinical course of leukemia. As ABCC4 carries the PEL antigen, PEL phenotyping could be useful in the adjustment of an optimal drug dose and prevention of chemotherapy side effects in leukemia. Our findings are of immediate and important relevance in transfusion medicine and in a personalized medicine approach for chemotherapy treatment follow-up in leukemia. Furthermore, the exceptional natural "knockouts" of ABC transporters are highly valuable in understanding their function, not only in erythroid cells, but also in other cells or tissues.
Hermine:Novartis: Research Funding; Celgene: Research Funding; AB Science: Consultancy, Equity Ownership, Honoraria, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal