Treatment with CD19-directed chimeric antigen receptor T cell (CART19) therapy has resulted in unprecedented clinical outcomes and was FDA-approved in acute lymphoblastic leukemia and non-Hodgkin B-cell lymphoma. However, its success in chronic lymphocytic leukemia (CLL) has been modest to date. An increasing body of evidence indicates that impaired CART cell fitness is the predominant mechanism of the relative dysfunction in CLL. The immunosuppressive microenvironment in CLL is well known and in part may be related to the abundance of circulating extracellular vesicles (EVs) bearing immunomodulatory properties. We hypothesized that CLL-derived EVs contribute to CART cell dysfunction.
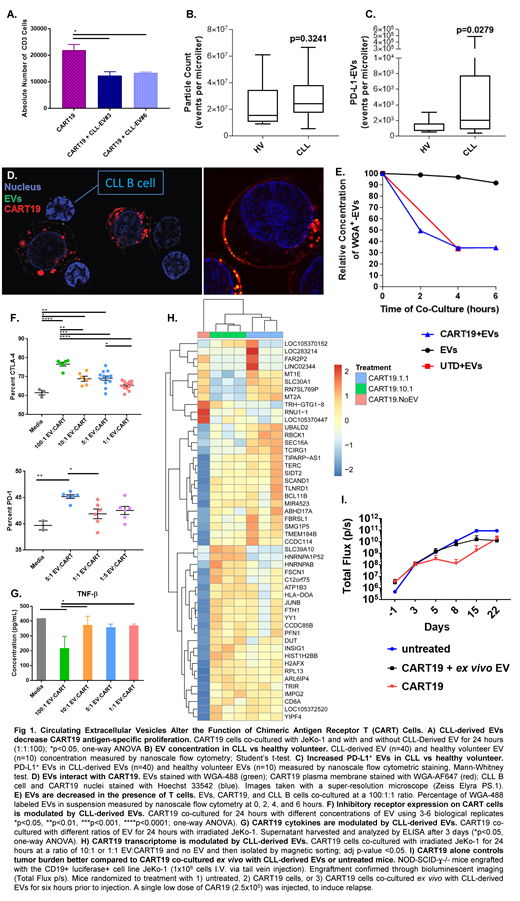
In this study, we aimed to investigate the interaction between circulating EVs isolated from CLL patient plasma (designated as CLL-derived EVs) and CART19 cells. We enumerated and immunophenotyped circulating EVs from platelet free plasma in untreated patients with CLL. We determined their interaction with CART19 cells using second generation, 41BB co-stimulated, lentiviral transduced CART19 cells generated in the laboratory from normal donors (FMC63-41BBζ CART cells). Our findings indicate that CLL-derived EVs impair normal donor CART19 antigen-specific proliferation against the CD19+ mantle cell lymphoma cell line Jeko-1 (Figure 1A). Next, we characterized CLL-derived EVs using nanoscale flow cytometric analysis of surface proteins and compared to healthy controls. Although the total EV particle count was not different between CLL and healthy controls (Figure 1B), there were significantly higher PD-L1+ EVs in patients with CLL (Figure 1C).
Based on these results, we sought to assess the physical interaction between CLL-derived EVs and CART cells from normal individuals. When CLL-derived EVs were co-cultured with CART19 and CLL B cells and imaged with super-resolution microscopy, EVs were localized at the T cell-tumor junction (Figure 1D). Furthermore, CLL-derived EVs are captured by T cells as indicated by a significant reduction in the absolute count of EVs when co-cultured with resting T cells (Figure 1E).
Having demonstrated that 1) there is an excess of PD-L1+ EVs in patients with CLL (Figure 1C) and 2) CLL-derived EVs physically interact with CART cells (Figures 1D-E), we sought to establish their functional impact on CART19 cells. Here, CART19 cells were stimulated with irradiated CD19+ JeKo-1 cells at a 1:1 ratio in the presence of increasing concentrations of CLL-derived EVs. There was a significant upregulation of inhibitory receptors such as PD-1 and CTLA-4 on the T cells (Figure 1F). This is associated with a reduction in CART effector cytokines (i.e., TNFβ) at higher concentrations of EVs (Figure 1G), suggesting a state of exhaustion in activated CART19 cells in the presence of CLL-derived EVs. This was further supported by transcriptome interrogation of CART19 cells. Here, CART19 cells were stimulated via 24-hour co-culture with the irradiated CD19+ cell line JeKo-1, in the presence of CLL-derived EVs at ratios of 10:1 and 1:1 EV:CART19 and then isolated by magnetic sorting. RNA sequencing of these activated CART19 cells indicated a significant upregulation of AP-1 (FOS-JUN) and YY1 (Figures 1H), known critical pathways in inducing T cell exhaustion.
Finally, to confirm the impact of CLL-derived EVs on CART19 functions in vivo, we used our xenograft model for relapsed mantle cell lymphoma. Here, immunocompromised NOD-SCID-ɣ-/- mice were engrafted with the CD19+ luciferase+ cell line JeKo-1 (1x106 cells I.V. via tail vein injection). Engraftment was confirmed through bioluminescent imaging and mice were randomized to treatment with 1) untreated, 2) CART19 cells, or 3) CART19 cells co-cultured ex vivo with CLL-derived EVs for six hours prior to injection. A single low dose of CAR19 (2.5x105) was injected, to induce relapse. Treatment with CART19 cells that were co-cultured ex vivo with CLL-derived EVs resulted in reduced anti-tumor activity compared to treatment with CART19 alone (Figure 1I).
Our results indicate that CLL-derived EVs induce significant CART19 cell dysfunction in vitro and in vivo, through a direct interaction with CART cells resulting in a downstream alteration of their exhaustion pathways. These studies illuminate a novel way through which circulating and potentially systemic EVs can lead to CART cell dysfunction in CLL patients.
Cox:Humanigen: Patents & Royalties. Sakemura:Humanigen: Patents & Royalties. Parikh:Ascentage Pharma: Research Funding; Janssen: Research Funding; AstraZeneca: Honoraria, Research Funding; Genentech: Honoraria; Pharmacyclics: Honoraria, Research Funding; MorphoSys: Research Funding; AbbVie: Honoraria, Research Funding; Acerta Pharma: Research Funding. Kay:Agios: Other: DSMB; Celgene: Other: Data Safety Monitoring Board; Infinity Pharmaceuticals: Other: DSMB; MorphoSys: Other: Data Safety Monitoring Board. Kenderian:Humanigen: Other: Scientific advisory board , Patents & Royalties, Research Funding; Lentigen: Research Funding; Novartis: Patents & Royalties, Research Funding; Tolero: Research Funding; Morphosys: Research Funding; Kite/Gilead: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal